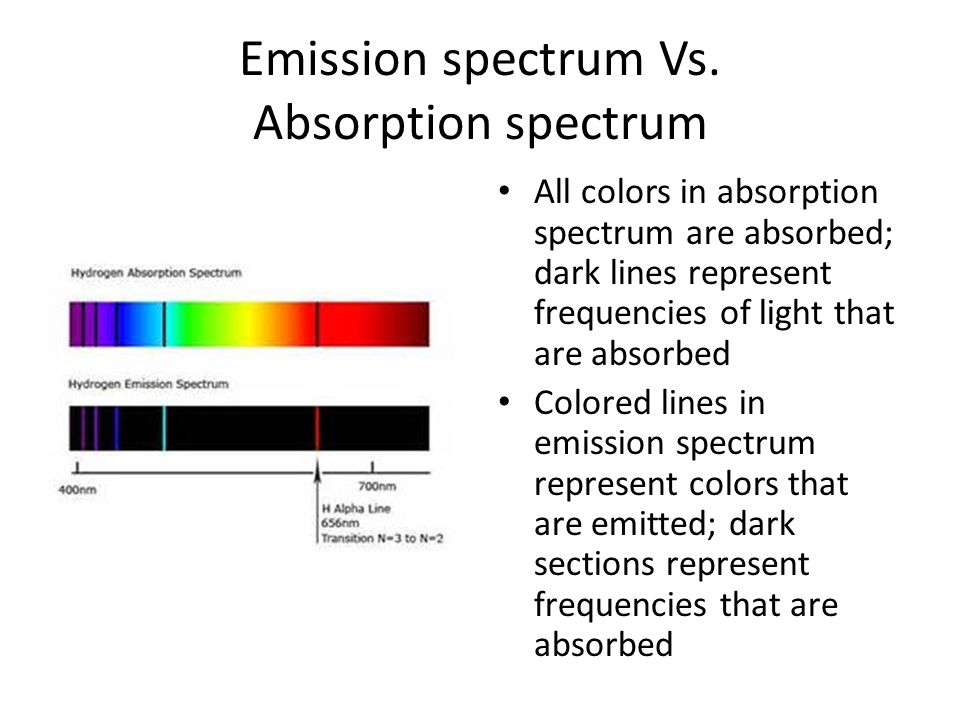
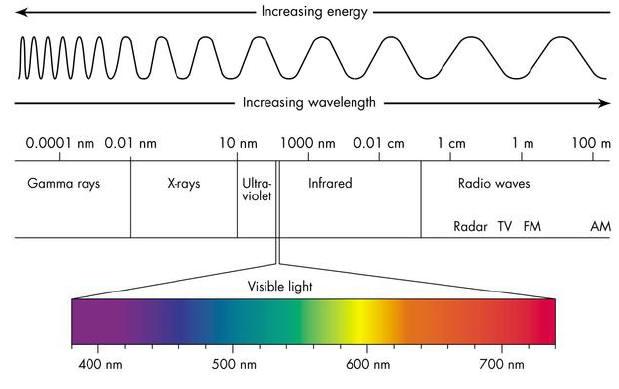
The full electromagnetic spectrum (Credit NASA's Imagine the Universe) The spectrum of the Sun appears as a continuous spectrum and is frequently represented as shown below This type of spectrum is called an emission spectrum because what you are seeing is the direct radiation emitted by the source In the case of the Sun, light is emittedThese lines make up the atomic emission spectrum of the atom being studied Each individual line represents a definite wavelength of light emitted by the excited atom The diffraction grating is a device which separates light into a spectrum of its individual wavelengths A prism behaves similarly Gratings are made by etching fine, parallel 2 Answers The Atomic Emission Spectra usefulness can be compared to the usefulness of a fingerprint the atomic emission spectra finger print makes a unique identifier Using this unique identifer, we have found out how hot the stars are, as well as what type of elements were they made of This led to the birth to modern astrophysics
Why Is Each Element S Emission Spectrum Unique Quora
To the best of your knowledge how do you think this emission spectrum is created
To the best of your knowledge how do you think this emission spectrum is created-Alright, so, that energy difference, if you do the calculations, that turns out to be the bluegreen line in your line spectrum So, I'll represent the light emitted like that And, if an electron fell from the 5th energy level down to the 2nd energy level, that corresponds to the blue line that you see on the line spectrum Teach Using the Lived Experiences of Your Students By Rebecca Alber close modal In education, we discuss the importance of using students' prior knowledge What is also important is learning about the "lived experiences" of the children we teach and connecting those experiences to the learning at hand



1
Intelligence is hard to come by these days We've created a short list of questions to test your intelligence when dealing with average everyday situations Take the intelligence test and we'll tell you how you compare to the rest of the world Looking up the answers is kind of cheating Observe your students closely, and value your reallife experience of diversity over the textbook version David Kolb created a fourstep model for really understanding the needs of a particular student group He starts with concrete experience, adds reflective observation and then moves to abstract conceptualization and active experimentation "What motivates you?" This interview question is trickier than you might imagine There is no one right way to answer it, but there are a number of replies to avoid With this guide, we'll cover how to best answer the what motivates you interview question, what not to do, and advice on more specific situations
Chemistry Bohr Model of the Atom Atoms and Electromagnetic Spectra 1 Answer B M It is created when the electrons in an atom return from a higher (excited) state to lowerAn emission line will appear in a spectrum if the source emits specific wavelengths of radiation This emission occurs when an atom, element or molecule in an excited state returns to a configuration of lower energy Since every atom, element and molecule has a unique set of energy levels, the emitted photon ('packet' of radiation) has a discrete wavelength, and an energy equal How an Emission Spectrum Is Produced When an atom or molecule absorbs energy, electrons are bumped into a higher energy state When the electron drops to a lower energy state, a photon is released equal to the energy between the two states What causes the bright lines in the emission spectrum?
Sample answers 1 I believe that my knowledge, attribute, skills, working experience, and inspiration that the position required makes me suitable candidate for this post I can confidently carry out the task mentioned in your job description Subscribe to Cold Call TikTok's parent company, ByteDance, was launched in 12 around the simple idea of helping users entertain themselves on their smartphones while on the Beijing Subway By May , TikTok operated in 155 countries and had roughly one billion monthly active users, placing it in the top ranks of digital platforms globallyThe emission spectrum of a fluorophore is the image of its absorption spectrum when the probability of the S 1 → S 0 transition is identical to that of the S o → S 1 transition If however, excitation of the fluorophore leads to a S o → S n transition, with n > 1, internal relaxation that will occur, so that the molecule reaches the first excited singlet state before emission, induces an



Emission Spectrum Of Hydrogen



The Emission Spectrum Of An Unknown Element Physics Forums
Strategies For Overcoming The DunningKruger effect is a type of cognitive bias in which people believe that they are smarter and more capable than they really are Essentially, low ability people do not possess the skills needed to recognize their own incompetence The combination of poor selfawareness and low cognitive ability leads them toSome good habits to have when thinking critically are being receptive to having your opinions changed, having respect for others, being independent and not accepting something is true until you've had the time to examine the available evidence, being fairminded, having respect for a reason, having an inquiring mind, not making assumptions, and always, especially, questioningA gas of hydrogen atoms will produce an absorptionline spectrum if it is between you (your telescopespectrograph) and a continuum light source, and an emissionline spectrum if viewed from a different angle If you were to observe the star (a source of white light) directly, you would see a continuous spectrum, with no breaks



Chemistry Of Fireworks Colors Munsell Color System Color Matching From Munsell Color Company




What Is The Difference Between Absorption And Emission Spectra Atomic Physics Youtube
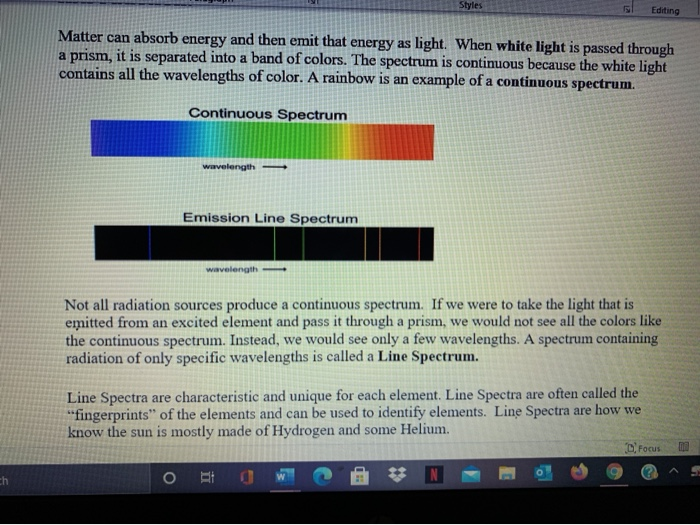
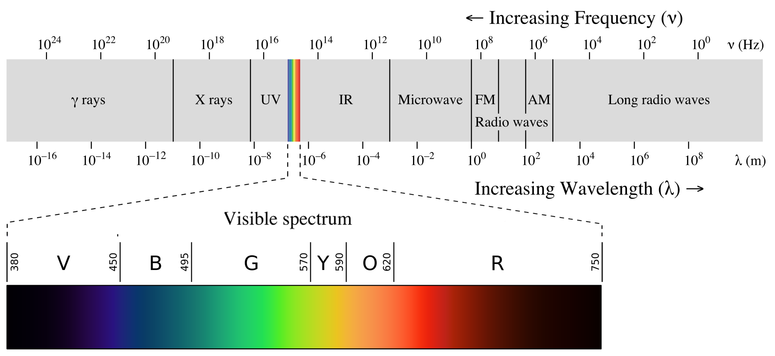
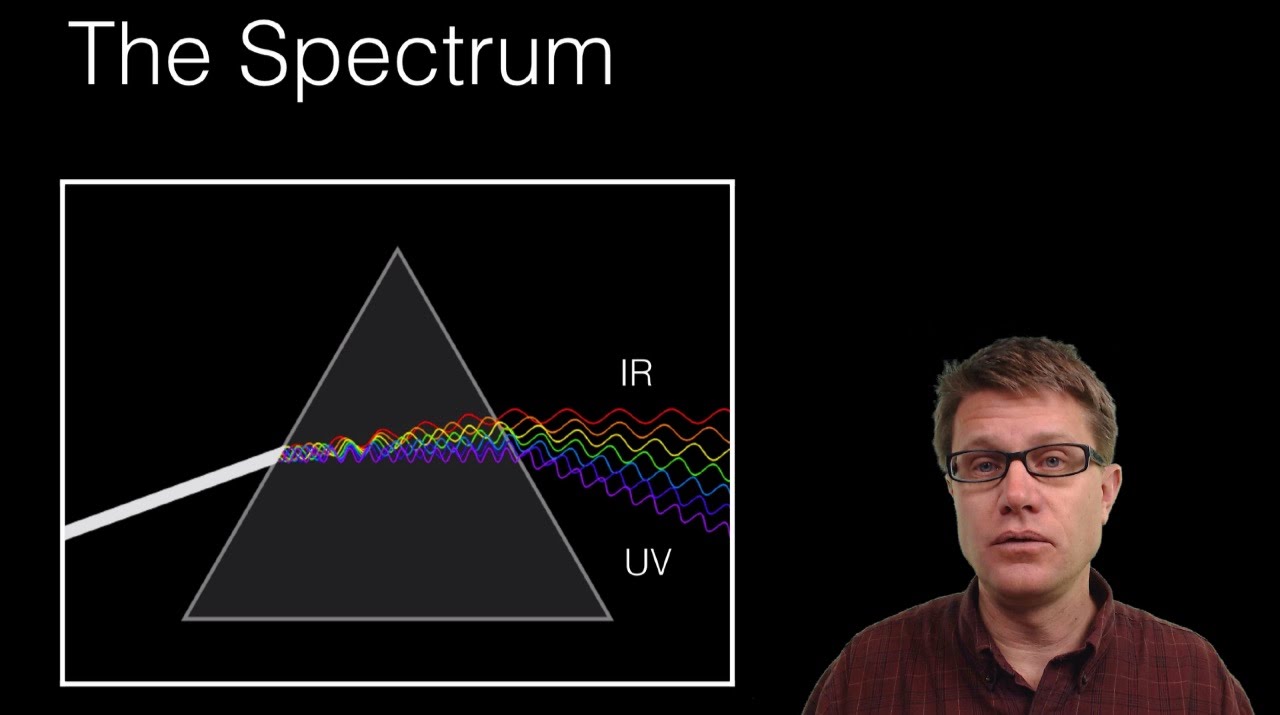
You're likely to discover unexpected gaps in your knowledge In psychology, we call this cognitive barrier the illusion of explanatory depth ItWash your hands with soap and water after handling the chemicals ***NEVER add more methanol to a watch glass that has already been burned!This activity will focus on the visible portion of the electromagnetic spectrum Background Information About 300 years ago, Sir Isaac Newton saw a beam of sunlight through a glass prism He discovered that light is made up of a spectrum of seven distinct visible colors This spectrum of colors always appears in the same order




Spectroscopy Definition Types Facts Britannica



Choosing Your Fluorescent Proteins For Multi Color Imaging
Use caution with the glassware after creating the flame, the watch glasses can remain hot for up to 10 minutes;1 Lab #14 EMISSION SPECTROSCOPY INTRODUCTION The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy, most commonly heat or electrical Since the frequency of light emitted under these conditions depends on the energies of the excited andHere is the emission spectra for a hydrogen atom In the black box, describe the process that is responsible for the emission of the red line and the violet line in the spectrum In the blue box, draw a diagram to illustrate your thinking Question Here is the emission spectra for a hydrogen atom In the black box, describe the process that is responsible for the emission of the red line
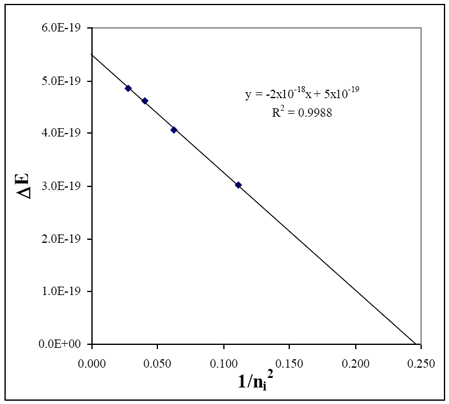



Lab 6 Quantum States For The Visible Hydrogen Atomic Emission Spectrum
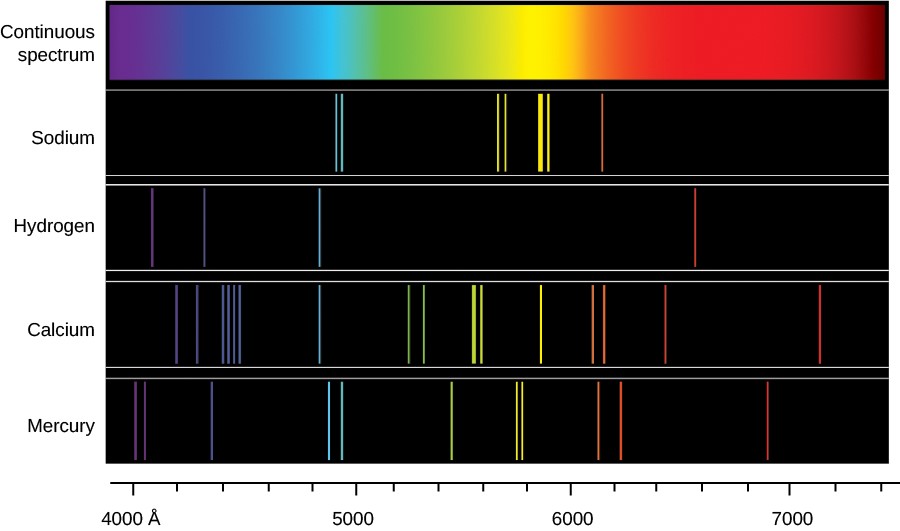



Spectroscopy In Astronomy Astronomy
Emission spectrum a graph of the number of xray photons and the range of energies the photons possess at a given exposure setting affected by changing several parameters kVp, mAs, target material, filtration, voltage waveformAdjusting the process allows you to construct a lesson that supports individual learners to meet their learning outcomes in a way that suits their specific needs Differentiating a lesson by adjusting the product When you adjust the product of a lesson, you are changing the specific success criteria for students to demonstrate what they have learned Use the sample questions and "Best Answers" listed at the end of this article to help you prepare your own personalized responses How to Answer Interview Questions About Leadership Prepare for interview questions about leadership by thinking about the leadership skills that are most important for the position




X Ray Emission Spectrum An Overview Sciencedirect Topics




Atomic Spectra Emission Spectrum Absorption Spectra Detailed Explanation With Videos
Explanation of the Emission Spectrum Max Planck presented a theoretical explanation of the spectrum of radiation emitted by an object that glows when heated He argued that the walls of a glowing solid could be imagined to contain a series of resonators that oscillated atQuora is a place to gain and share knowledge It's a platform to ask questions and connect with people who contribute unique insights and quality answers This empowers people to learn from each other and to better understand the worldAn emission spectrum is the range or array of wavelengths (spectra) obtained when the light emitted by a substance is passed through a prism and examined directly with a spectroscope Now let's define the line emission spectrum a spectroscope splits the emitted light into different wavelengths and gives a discontinuous spectrum in the form of
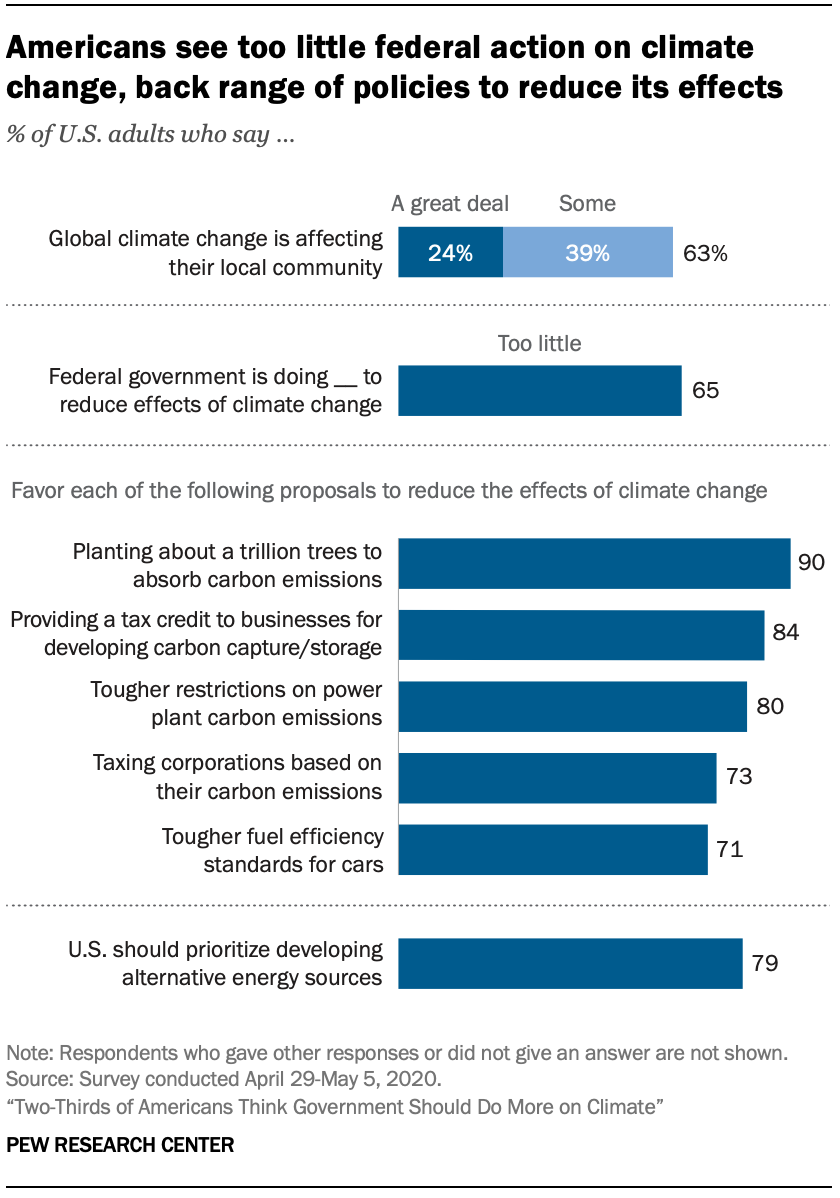



Two Thirds Of Americans Think Government Should Do More On Climate Pew Research Center




Between The Gender Lines The Science Of Transgender Identity Science In The News
21 21 51 Benefits of Critical Thinking Daily life Helps us avoid making foolish decision Helps us become a good citizen capable of making good decisions on important social, political and economic issues Helps us in developing good thinking skill capable of examining our own assumptions and dogmas 22 22 6To accomplish this job, these atoms and molecules emit radiation in various regions of the electromagnetic spectrum This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as an emission spectrum Absorption Spectrum We observe that when a ray of white light falls on a prism it experiences refraction twiceThe emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy state The photon energy of the emitted photon is equal to the energy difference between the two states There are many possible electron transitions for




Spectroscopy Interaction Of Light And Matter Article Khan Academy




How Exactly Does Carbon Dioxide Cause Global Warming You Asked
The 3 Best Books on SelfReflection and Introspection There are many books out there on selfreflection, selfawareness, and introspection, but we recommend the books below as resources to help you start your journey 1 Question Your Life Naikan SelfReflection and the Transformation of Our Stories – Gregg Krech This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum It can be defined as The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an atom or molecule making a transition from a high energy state to a lower energy called an emission spectrum because the light is emitted from the element Alternatively, if you shine white light through a gaseous element and then let the light pass through a prism you see dark lines in the continuous spectrum This is called an absorption spectrum because the gas is absorbing light at speci c wavelengths




5 1 Light Quantized Energy Flashcards Quizlet



Temperature Dependent Absorption And Emission Spectra Of Tm Caf2
Answer (1 of 2) You said you know the theory, but what you might not understand is how much a good experimenter must really know theory in depth No doubt somebody will post the equipment details, or you can google them yourself, but I am going toHow smart are you? 3 Store your data in a place where you can remember A folder stored in a drawer is the best idea, but other ideas are good too Also, below is how things work with radio waves (the y in the picture is short for gamma rays) Do this step last after every section



2
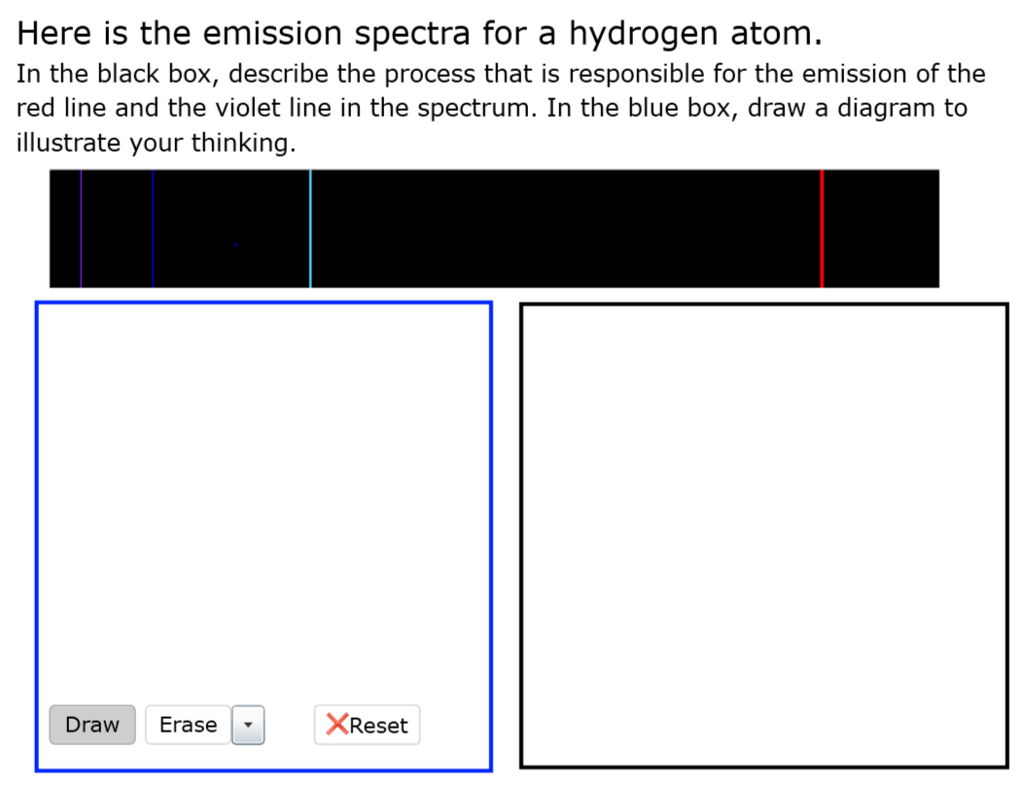



Here Is The Emission Spectra For A Hydrogen Atom In Chegg Com
How is a line emission spectrum created?Then as the electrons in the atoms fall back down, they emit electromagnetic radiation (light) The amount of light emitted at different wavelengths, called the emission spectrum, is shown for a discharge tube filled with hydrogen gas in Figure 126 below Only certain wavelengths (ie colours) of light are seen, as shown by the lines in the pictureAn atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains The Figure below shows the atomic emission spectrum of hydrogen Figure 2 When light from a hydrogen gas discharge tube is passed through a prism, the light is split into four visible




Effects Of Working Medium Gases On Emission Spectral And Temperature Characteristics Of A Plasma Igniter
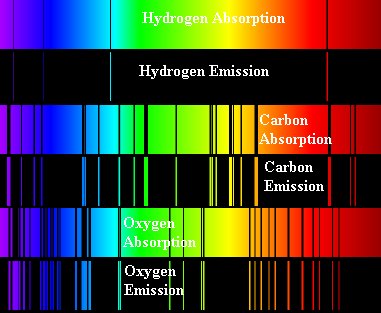



Astronomy Notes 4 Light And Telescopes
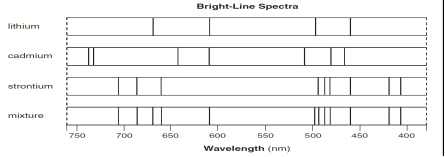
Herschel's discovery of emission spectra from heated gas was studied extensively in the 1800's It was realized that a heated gas emits a unique combination of colors, called emission spectrum, depending on its composition Example Helium gas in a discharge lamp Main idea put a large voltage across the gas It will break down and emit light As I say below, an electron cannot be absorbed, which is what I think you are implying above, but a photon, as the force carrier between electrons, can be absorbed and emitted I think there are duplicates for the other related questions in your post, so I And so if you feel like it's going to be successful, that has a little bit to do with selfconfidence, it has a little bit to do with the type of feedback you're getting from the people around you, a little bit to do with whether you've had a mentor who's been there before and says, "Hey, you could do this too" And if we think women may be a little bit less plugged into some of those




Using Spectra To Measure Stellar Radius Composition And Motion Astronomy
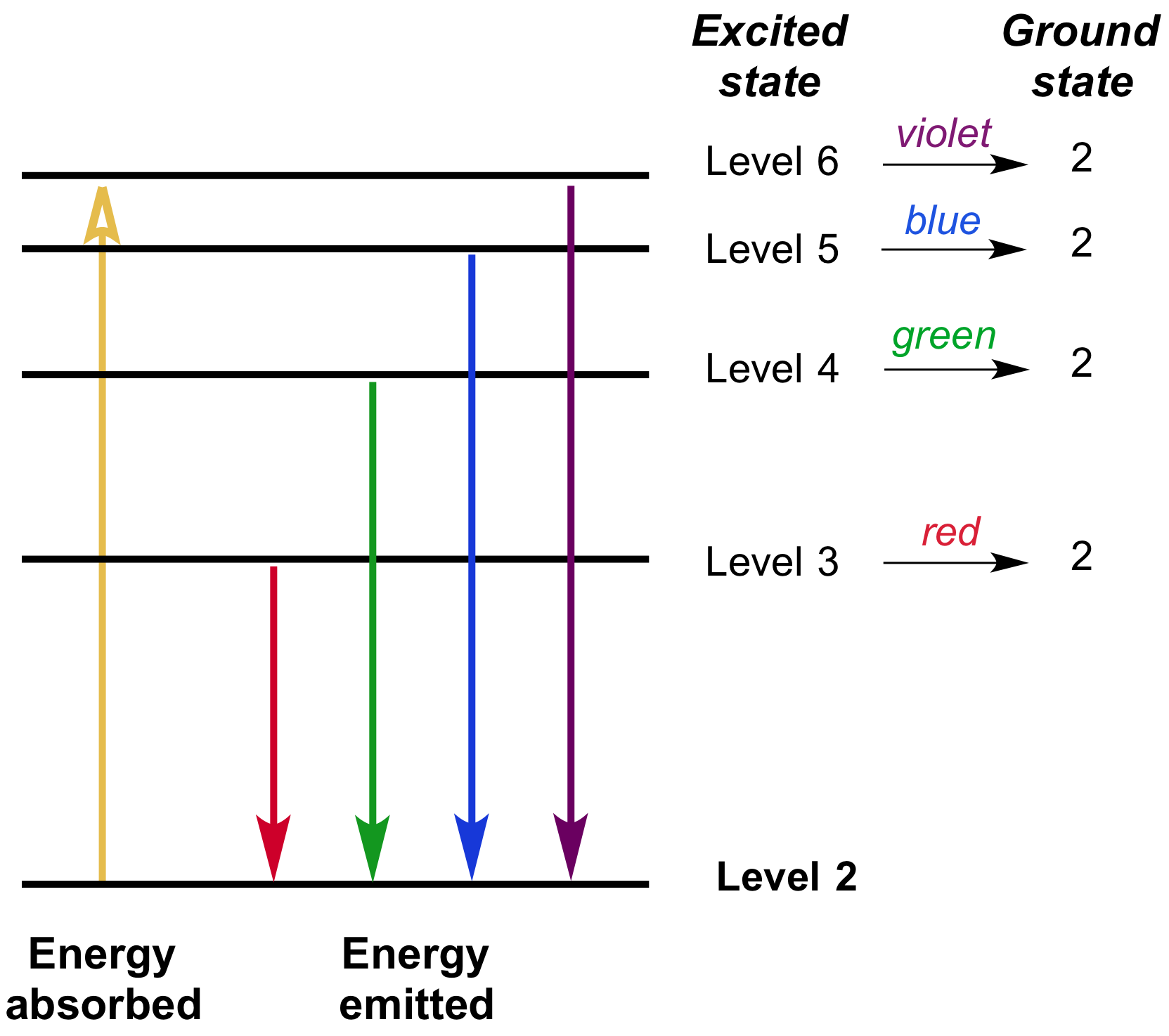



Spectroscopy Interaction Of Light And Matter Article Khan Academy
In astronomy, the emission spectrum generally refers to the spectrum of a star, nebula, or another body How an Emission Spectrum Is Produced When an atom or molecule absorbs energy, electrons are bumped into a higher energy state When the electron drops to a lower energy state, a photon is released equal to the energy between the two statesWhat you would see is a small part of the hydrogen emission spectrum Most of the spectrum is invisible to the eye because it is either in the infrared or the ultraviolet The photograph shows part of a hydrogen discharge tube on the left, and the three most easily seen lines in the visible part of the spectrum on the right
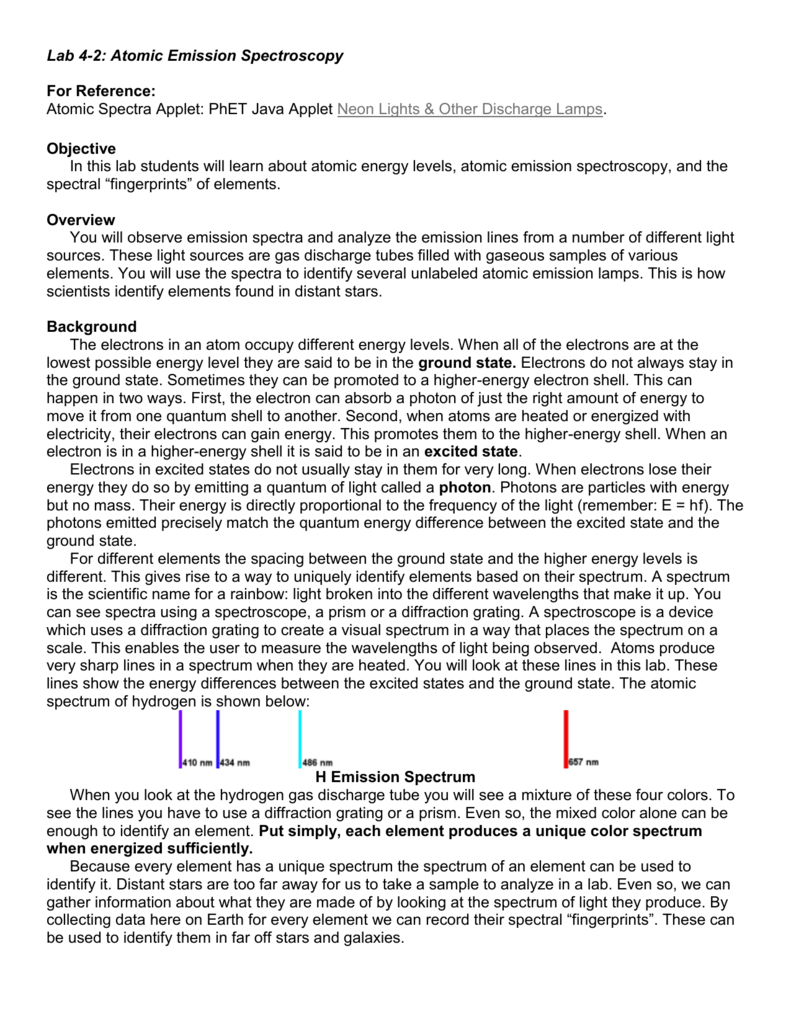



Lab 4 2 Atomic Emission Spectroscopy




Thermal Emission And Reflected Light Spectra Of Super Earths With Flat Transmission Spectra Iopscience



Measurement Of 3 Photon Excitation And Emission Spectra And Verification Of Kasha S Rule For Selected Fluorescent Proteins Excited At The 1700 Nm Window
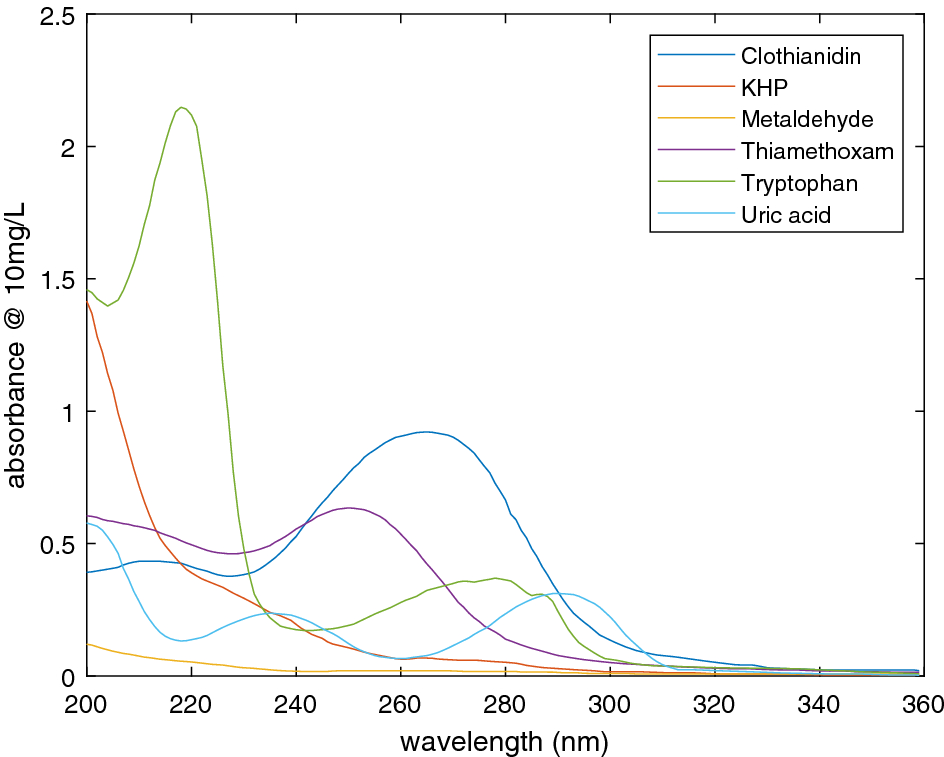



Ultraviolet Absorption Of Contaminants In Water Scientific Reports



How Do Scientists Determine The Chemical Compositions Of The Planets And Stars Astronomy Com
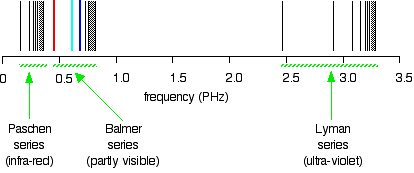



Hydrogen S Atomic Emission Spectrum Chemistry Libretexts




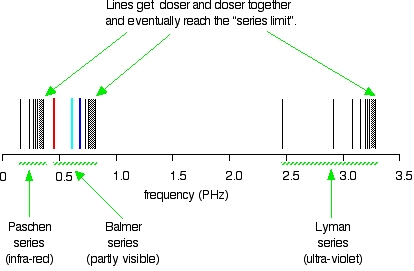
Formation Of Spectral Lines Astronomy
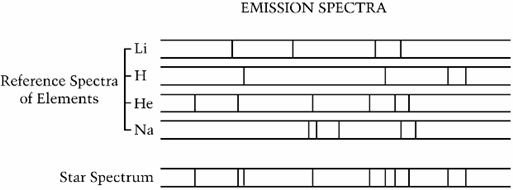



Naep 09 Science Sample Questions




Light Emission And Absorption Processes Britannica
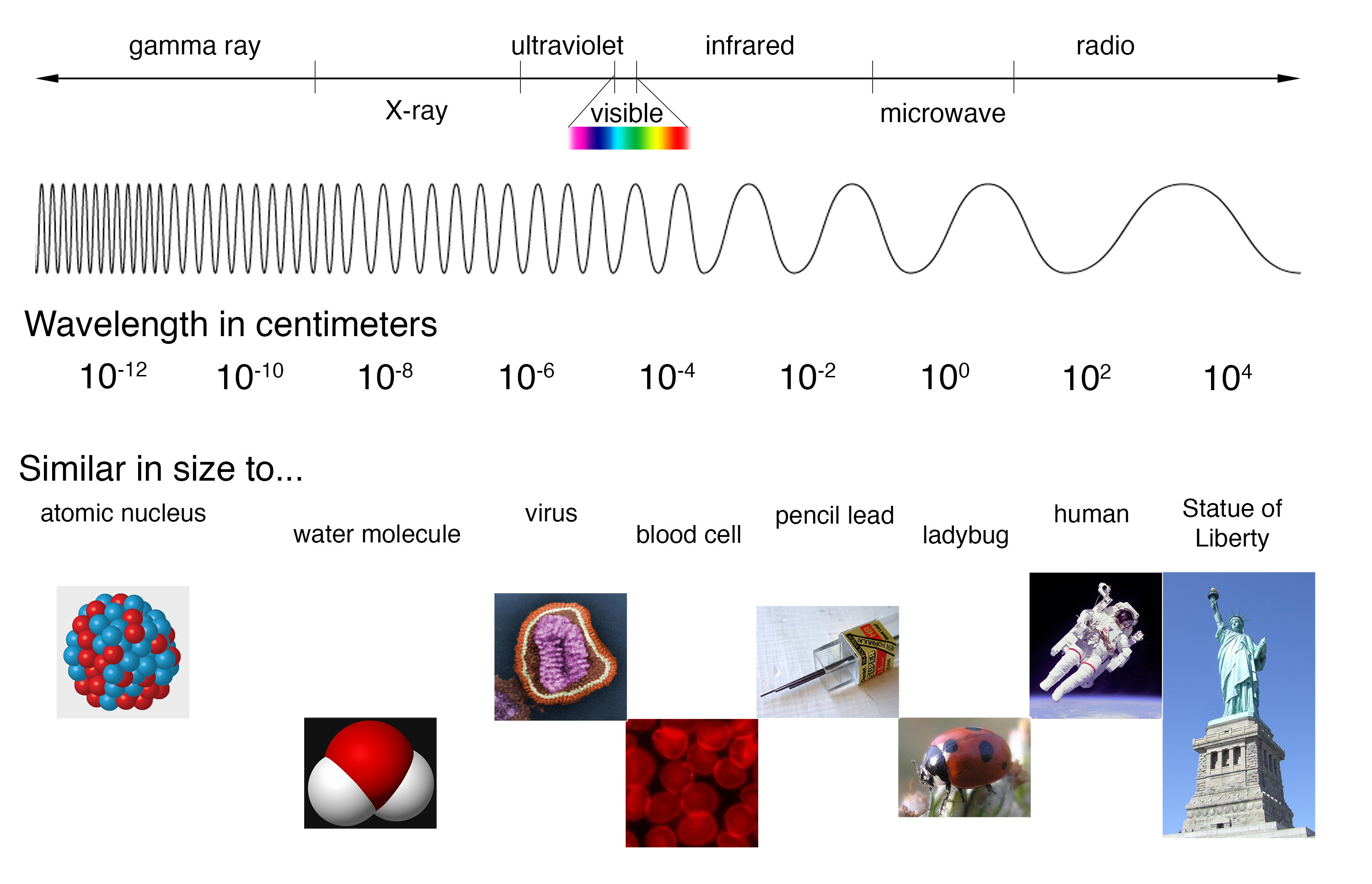



Imagine The Universe
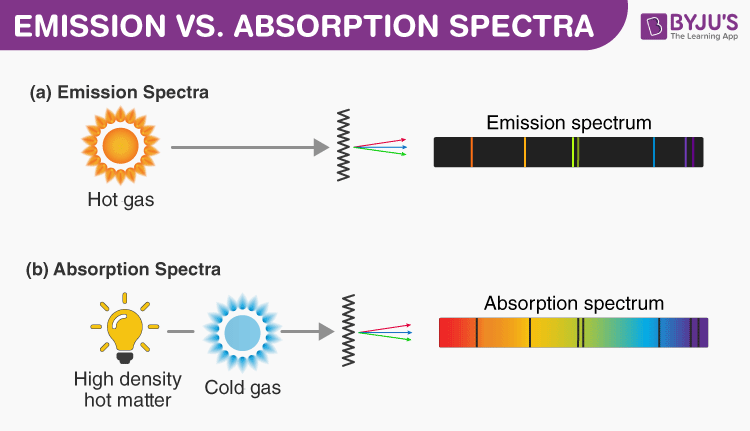



Difference Between Emission And Absorption Spectra Comparison Chart



Temperature Dependent Absorption And Emission Spectra Of Tm Caf2




Chapter 2 Section 5



Chart Which Countries Are Meeting Their Paris Agreement Goals Statista




How Do Scientists Determine The Chemical Compositions Of The Planets And Stars Astronomy Com



1




What Is The Difference Between Fluorescence Phosphorescence And Luminescence Enzo Life Sciences



How To Choose The Correct Wavelength In Icp Oes



Amplified Spontaneous Emission In Paper Scientific Reports



Solved A ă Ee2119 X A Ee b Ccddz bba bb Chegg Com




Hydrogen Emission Spectrum Spectroscopy Successive Ionisation Energy Patterns Related To Sub Shells And Group Of Periodic Table Gce A Level Revision Notes



Why Is Each Element S Emission Spectrum Unique Quora
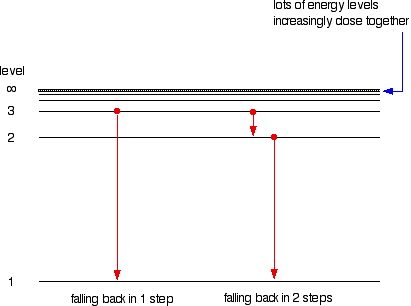



Atomic Hydrogen Emission Spectrum



Imagine The Universe



4 2 Understanding Atomic Spectra Chemistry Libretexts




Chapter 2 Section 5
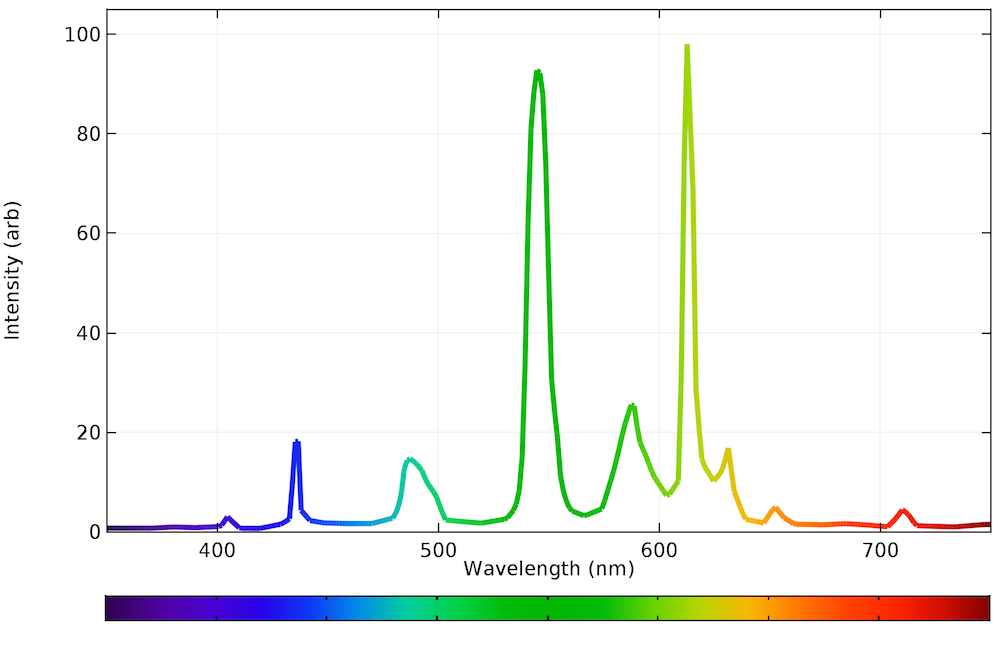



Calculating The Emission Spectra From Common Light Sources Comsol Blog
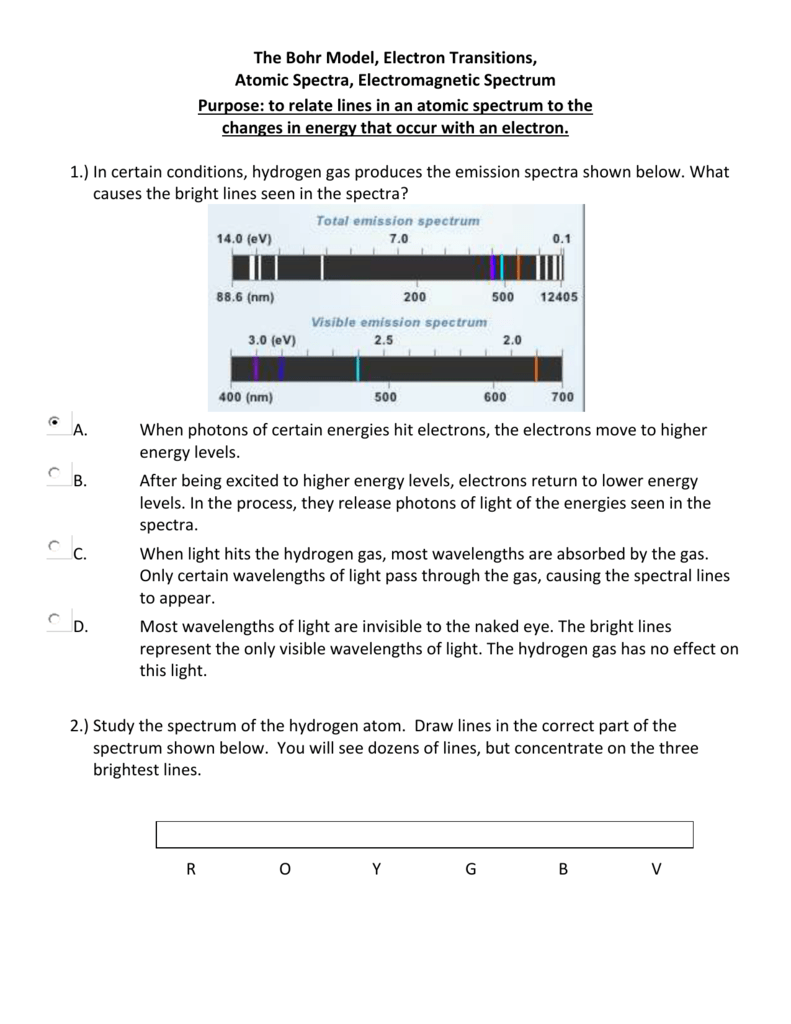



Atomic Spectra Electromagnetic Spectrum
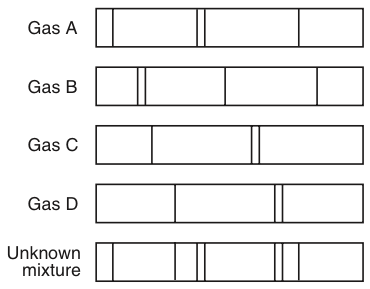



Energy Changes Atomic Emission Spectra Proprofs Quiz




Light Emission And Absorption Processes Britannica
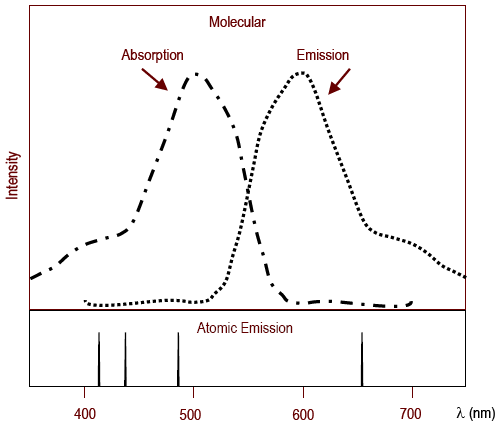



Lab 9 Determination Of Allura Red Concentration In Mouthwash




Emission Line Spectra
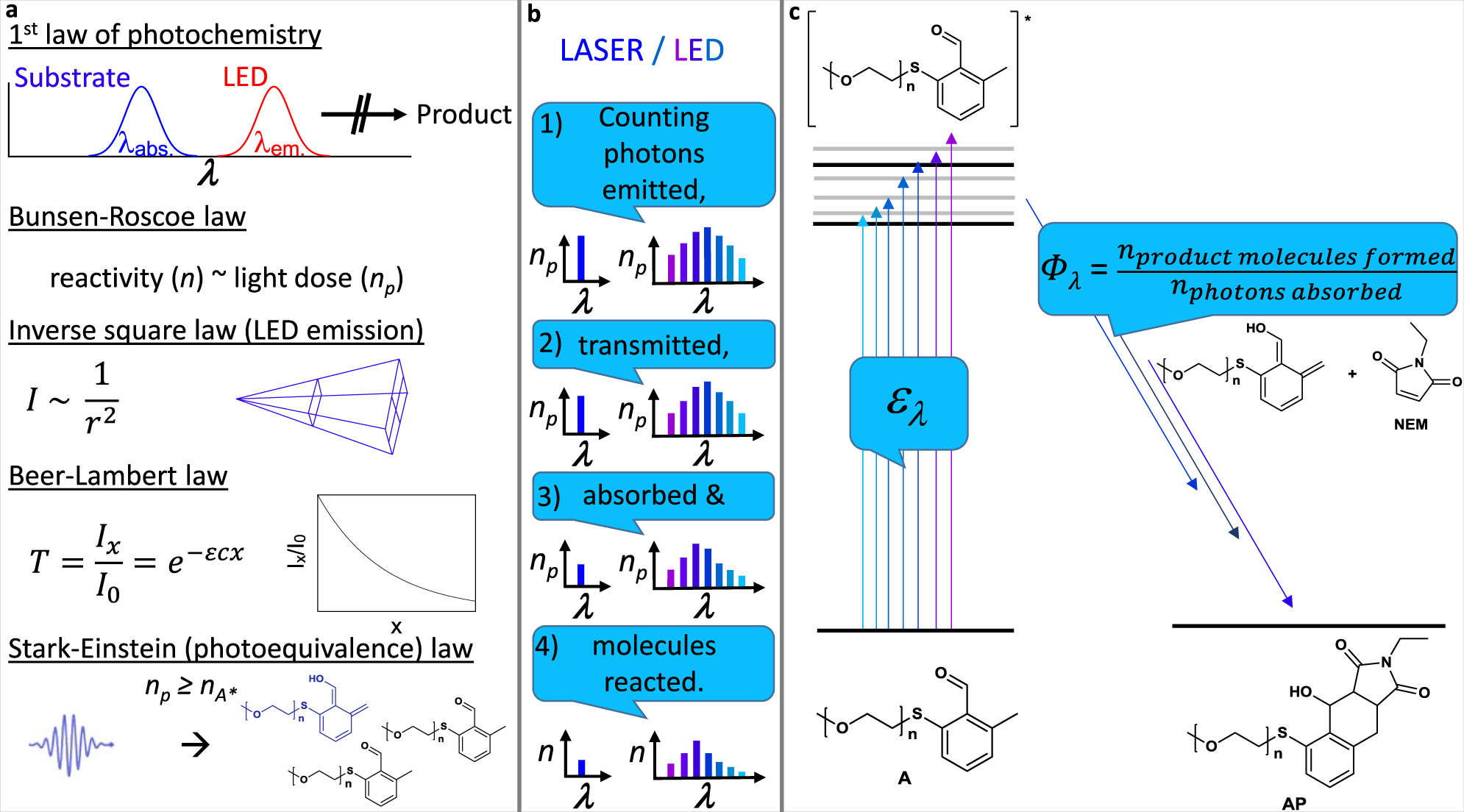



Predicting Wavelength Dependent Photochemical Reactivity And Selectivity Nature Communications



Atomic Emission Spectrum Make Up Lab Ppt Video Online Download



Buyer Beware This Led Bulb Sold As Germicidal Doesn T Emit Uv C Hackaday




Formation Of Spectral Lines Astronomy



Imagine The Universe



Using Flame Tests To Identify Unknowns
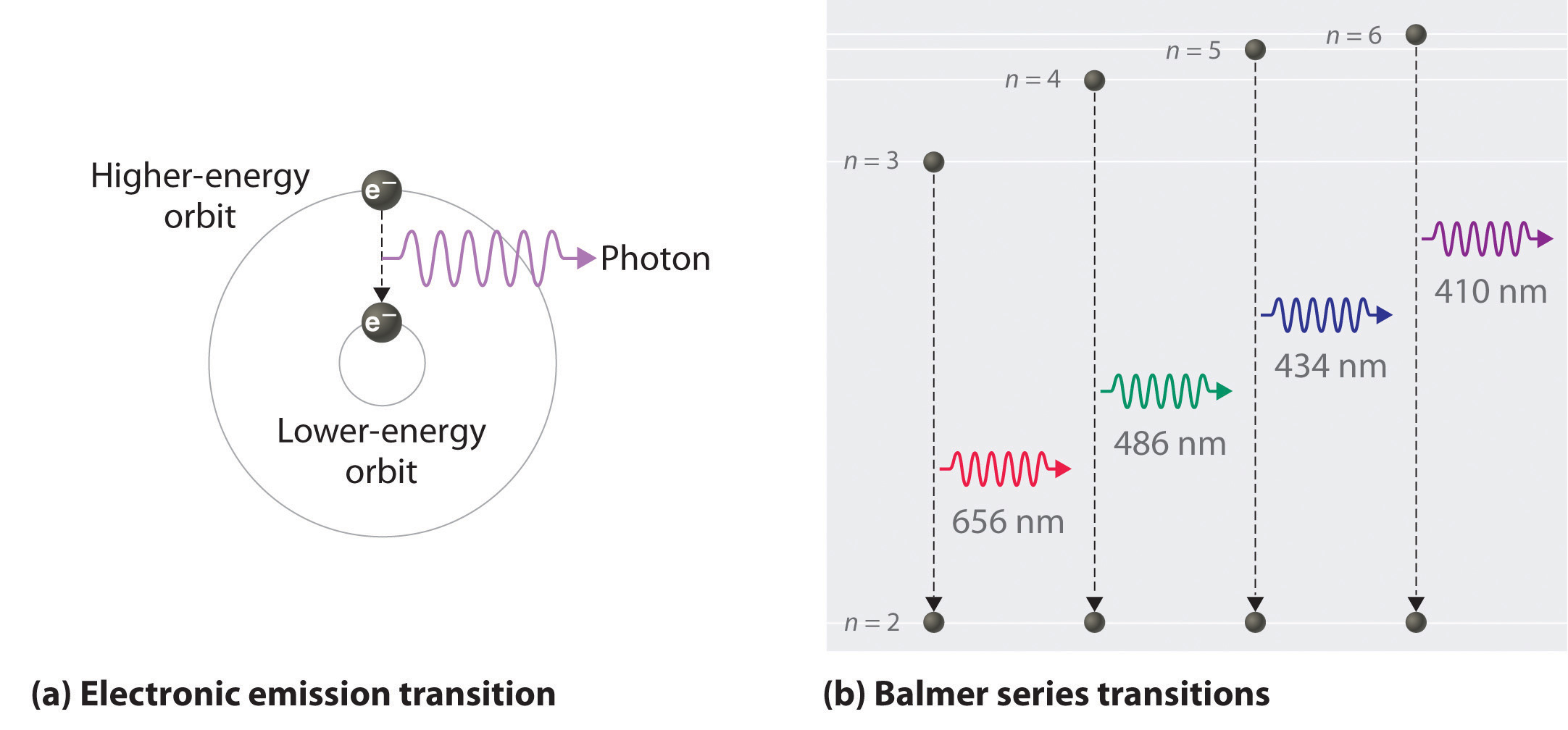



6 3 Line Spectra And The Bohr Model Chemistry Libretexts




Emission Spectrum Of Hydrogen Video Khan Academy



Calculating The Emission Spectra From Common Light Sources Comsol Blog
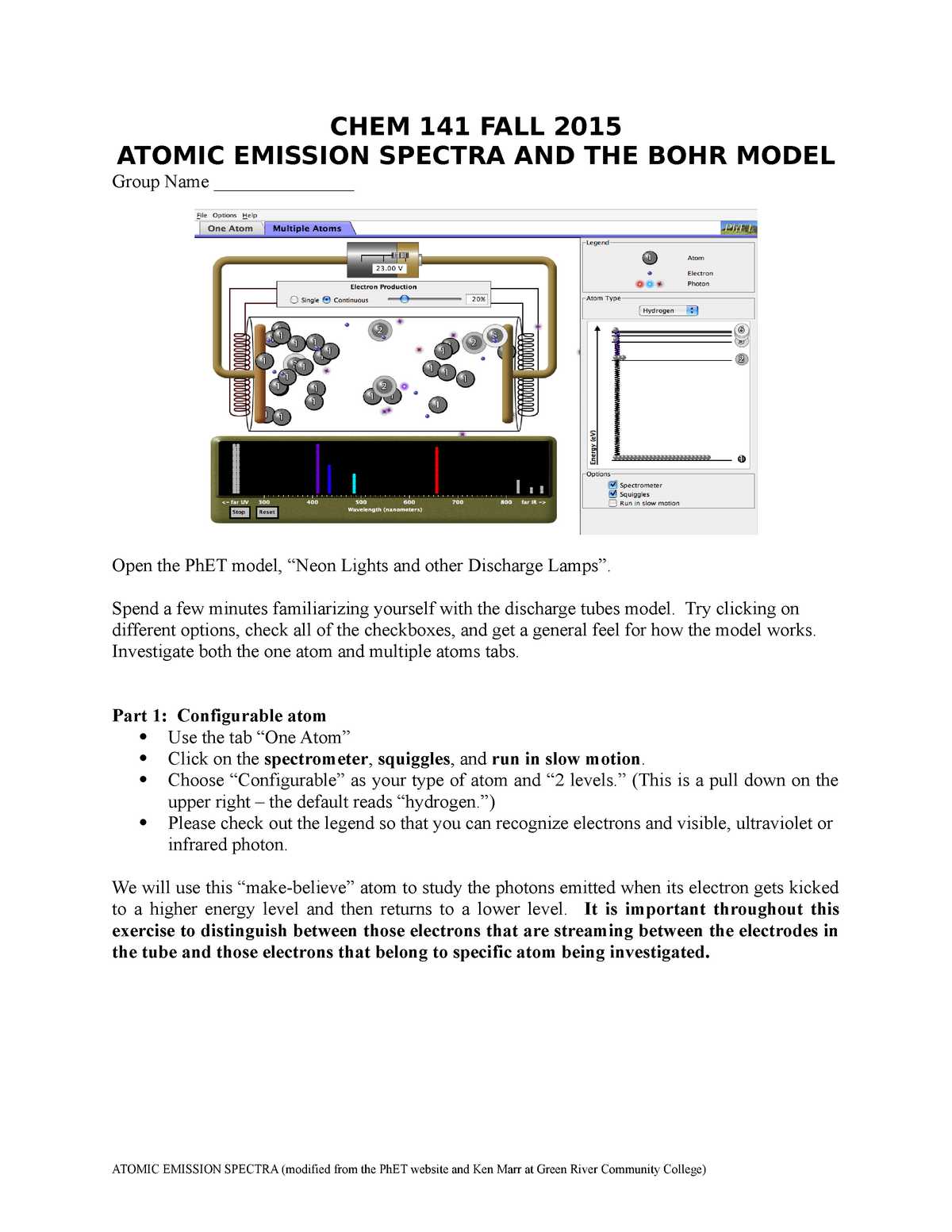



C3 Atomic Emission Spectra And Bohr Chem 141 General Chemistry I Studocu
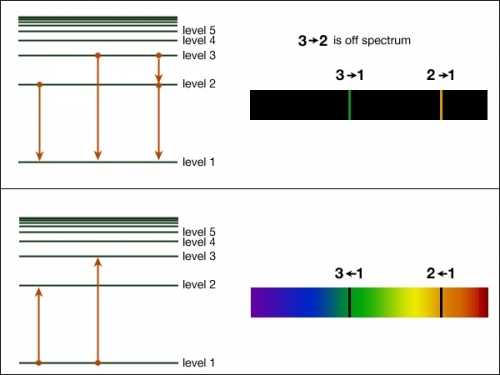



Kirchoff S Laws And Spectroscopy Astronomy 801 Planets Stars Galaxies And The Universe




Effects Of Working Medium Gases On Emission Spectral And Temperature Characteristics Of A Plasma Igniter
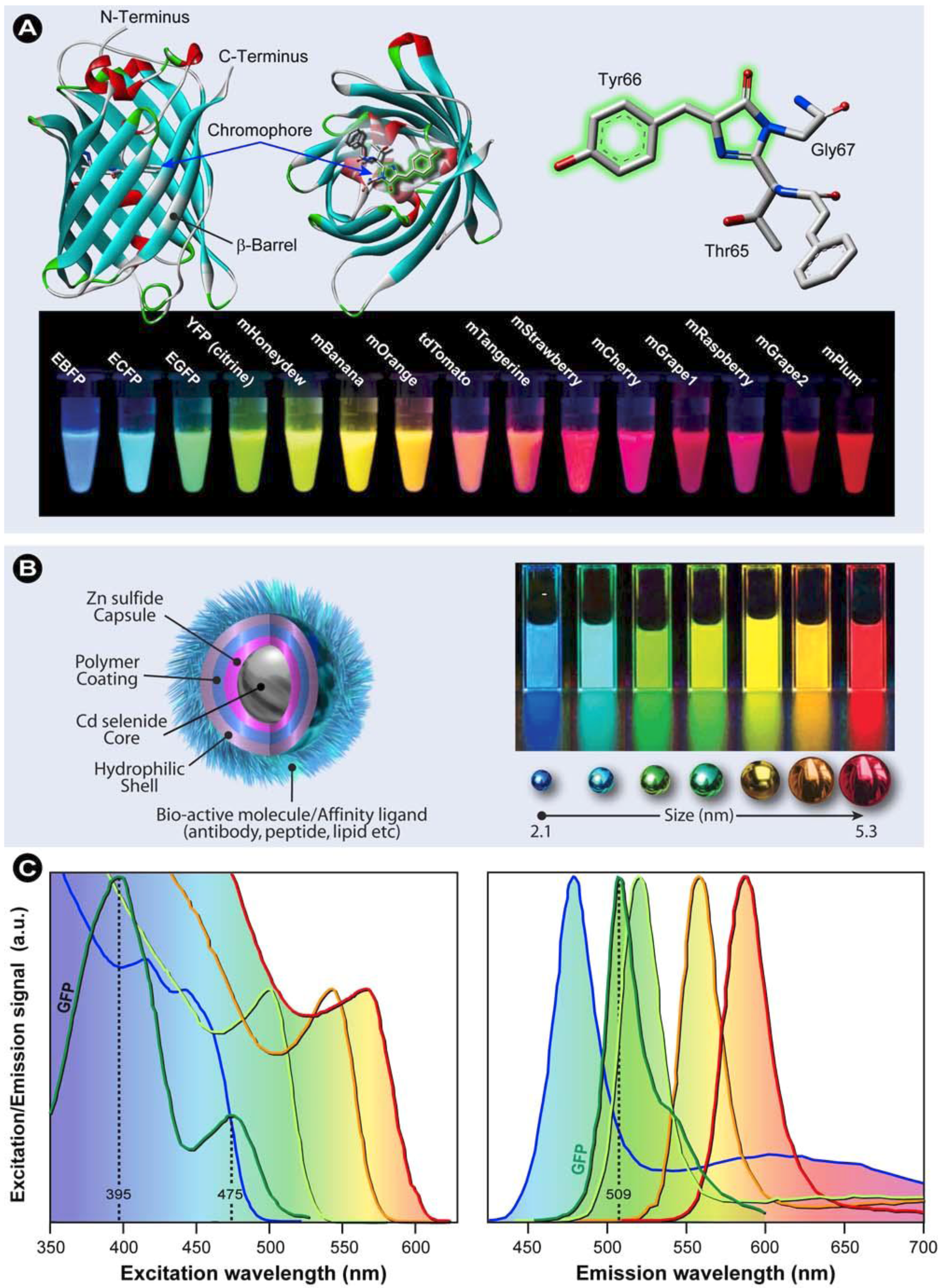



Molecules Free Full Text Advanced Fluorescence Microscopy Techniques Frap Flip Flap Fret And Flim Html



Why Do Molecules Show The Band Spectra Rather Than The Line Spectra Quora
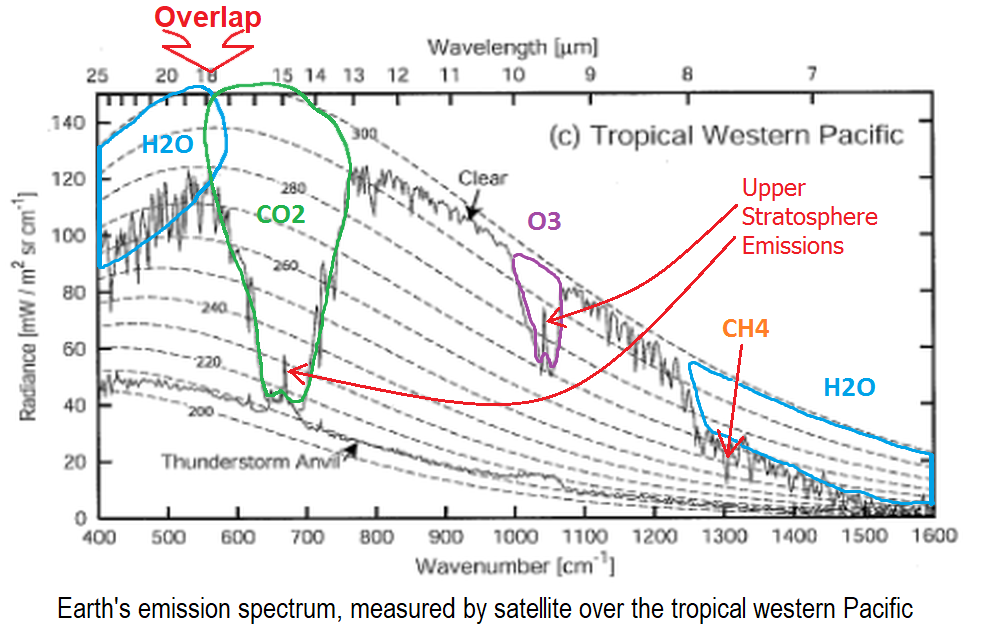



Doubling Co2 And Basic Physics Clive Best



3



2
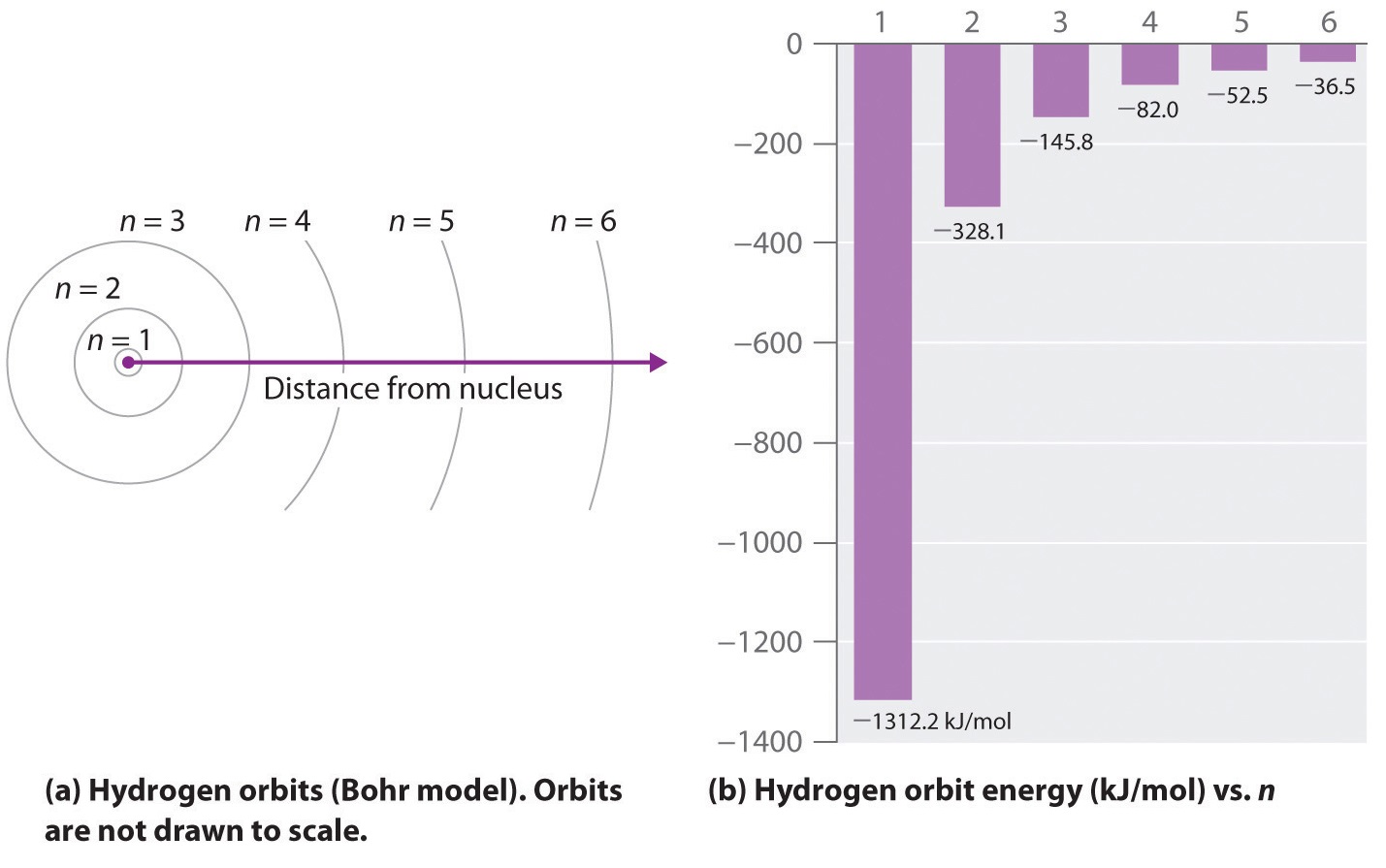



6 3 Line Spectra And The Bohr Model Chemistry Libretexts




Emission And Absorption Spectra Bohr Line Spectrum Examples Videos




Atomic Spectra Emission Spectrum Absorption Spectra Detailed Explanation With Videos




Astronomy 1600 Guelph Week 6 Unit 6 Light Spectra Flashcards Quizlet



1



Atoms And Periodic Trends For The Mcat Everything You Need To Know Shemmassian Academic Consulting



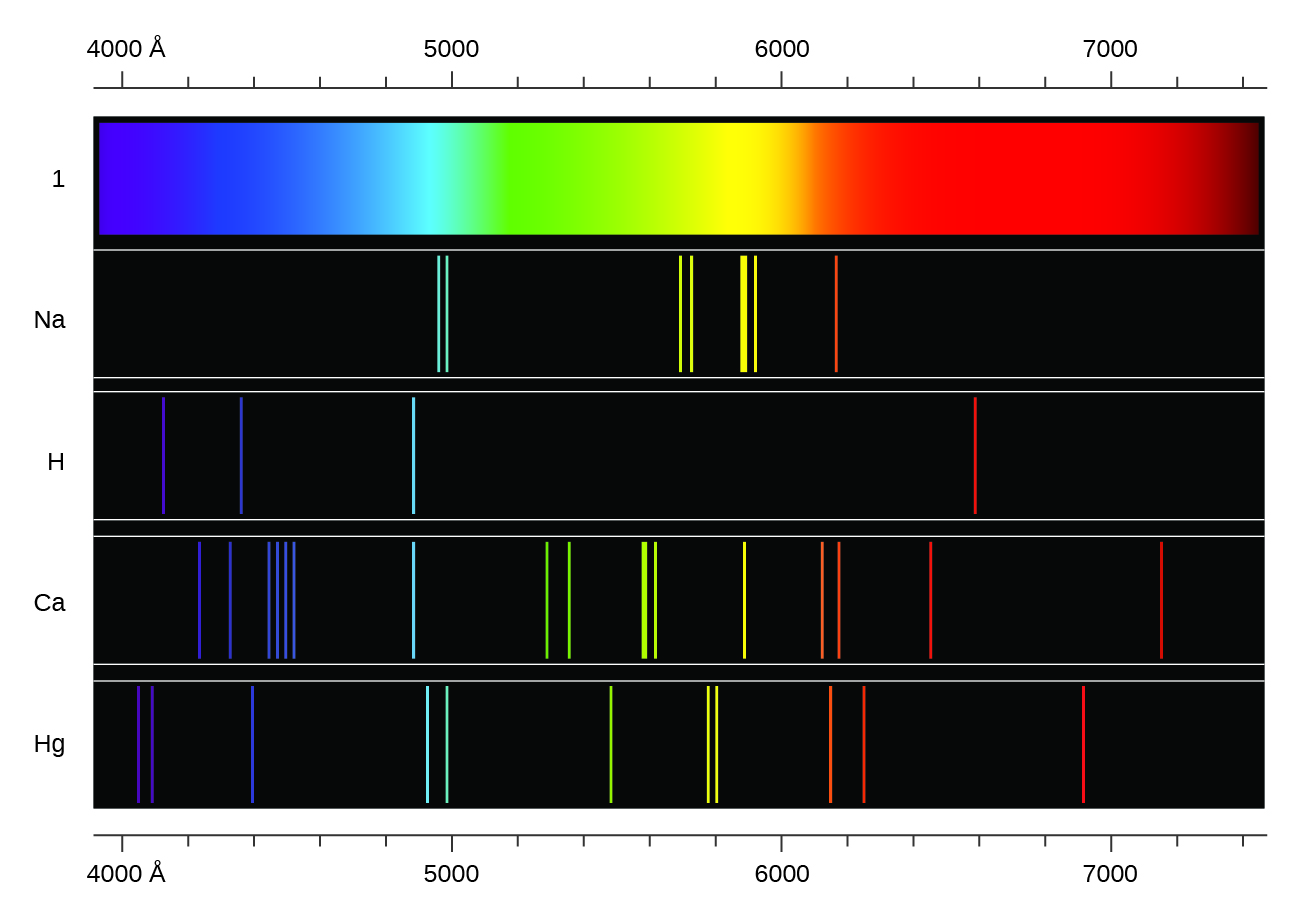
Flame Tests Causes Of Color



Emission And Absorption Spectra Youtube




Hydrogen Emission Spectrum Spectroscopy Successive Ionisation Energy Patterns Related To Sub Shells And Group Of Periodic Table Gce A Level Revision Notes




Light Ck 12 Foundation



6 1 Electromagnetic Energy Chemistry



Chemistry Of Fireworks Colors Munsell Color System Color Matching From Munsell Color Company



Hydrogen S Atomic Emission Spectrum Chemistry Libretexts




Emission And Absorption Spectra Bohr Line Spectrum Examples Videos




Absorption Spectroscopy Wikipedia




Emission Spectra Of Different Light Sources A Incandescent Tungsten Download Scientific Diagram
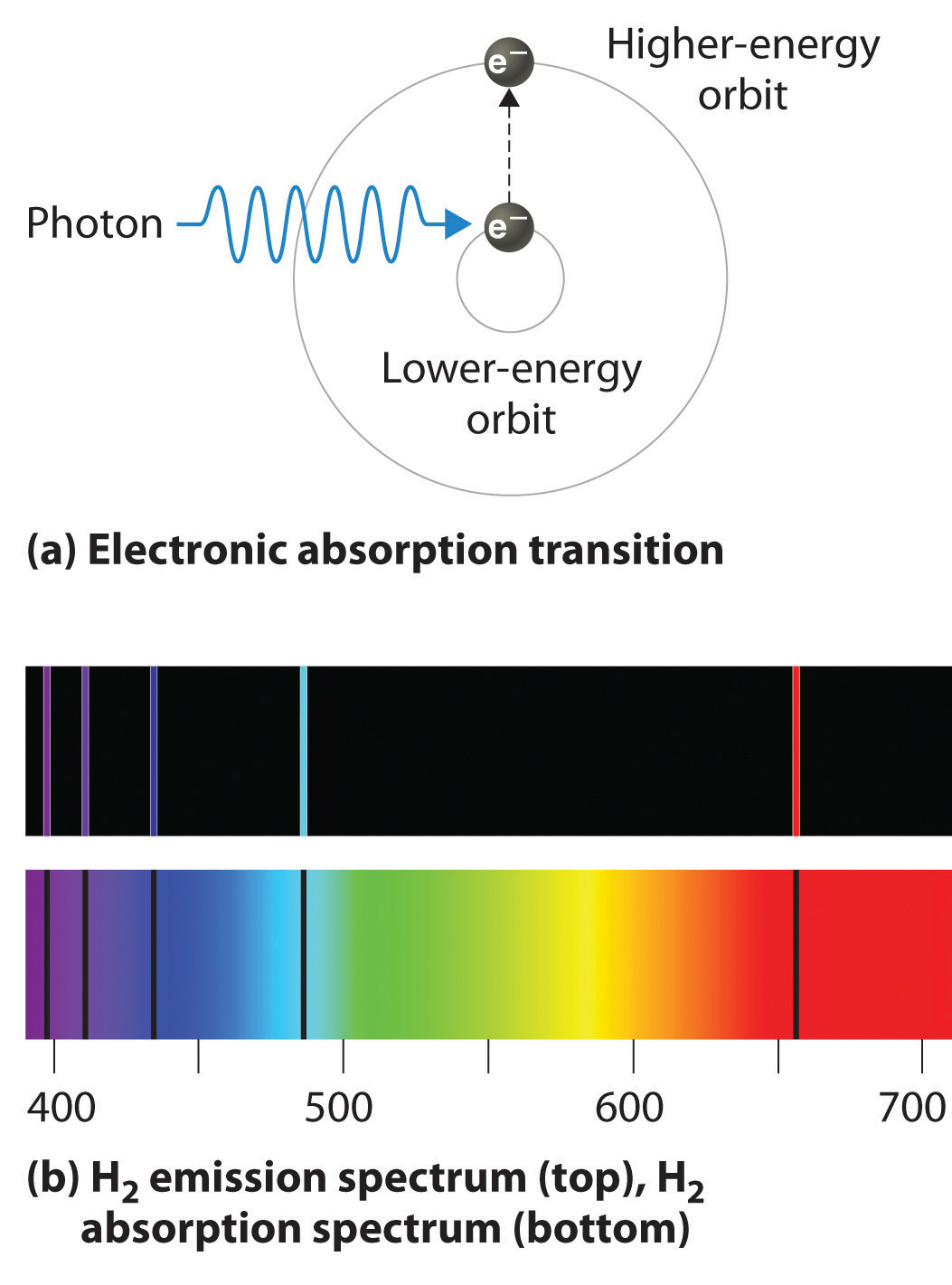



6 3 Line Spectra And The Bohr Model Chemistry Libretexts
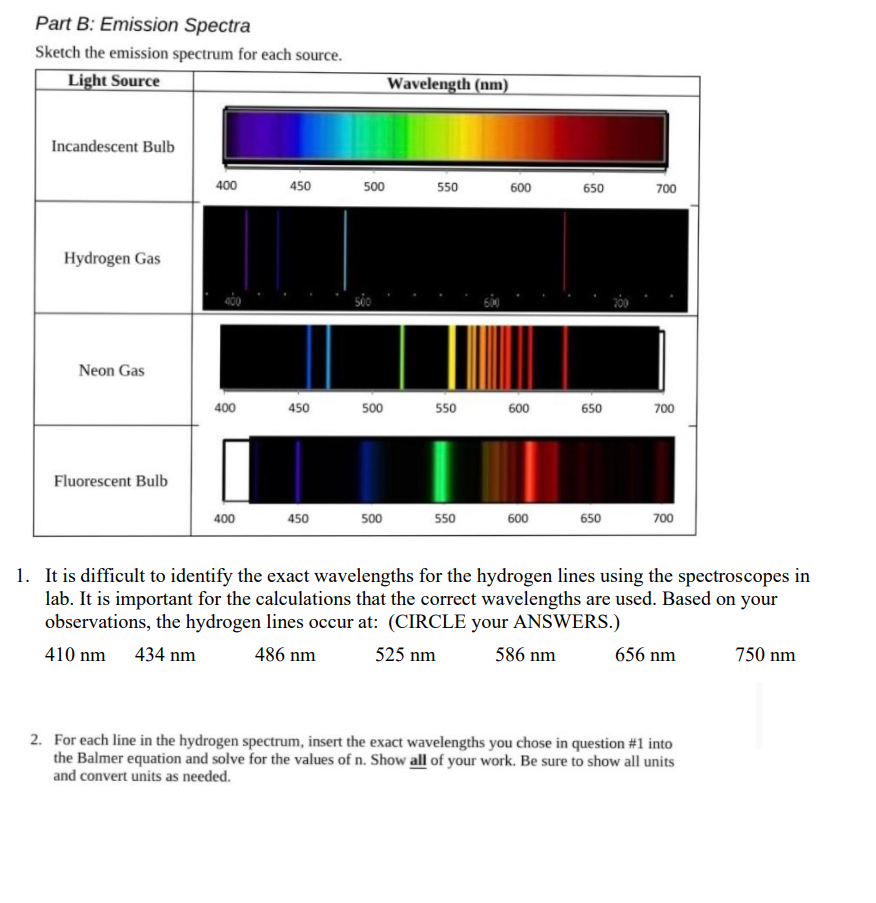



Solved Part B Emission Spectra Sketch The Emission Spectrum Chegg Com




How Does Spectroscopy Help Identify Elements
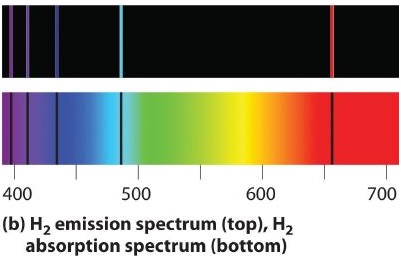



6 3 Line Spectra And The Bohr Model Chemistry Libretexts



1




Lab 7 Using Emission Spectra To Identify Pure Element
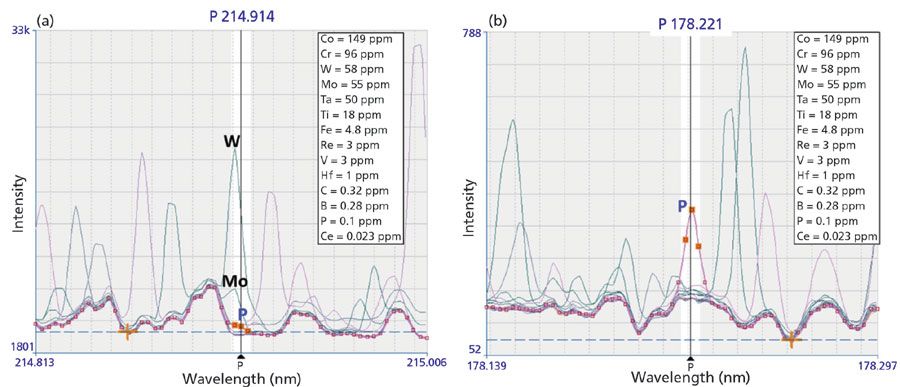



How To Choose The Correct Wavelength In Icp Oes
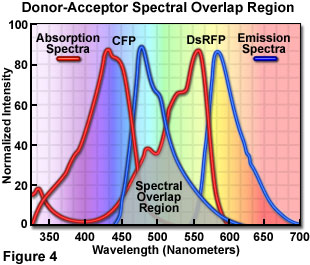



Fluorescence Resonance Energy Transfer Fret Microscopy Introductory Concepts Olympus Ls




X Ray Emission Spectrum An Overview Sciencedirect Topics




Spectra In The Lab



Emission Spectrum Of Hydrogen




Hydrogen Spectrum Activity Carolina Com




Absorption Spectroscopy Wikipedia
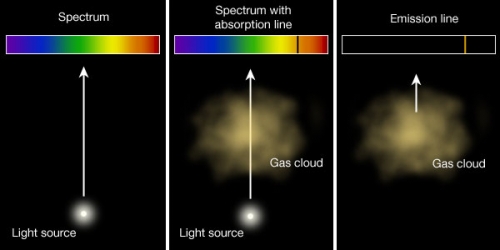



Kirchoff S Laws And Spectroscopy Astronomy 801 Planets Stars Galaxies And The Universe



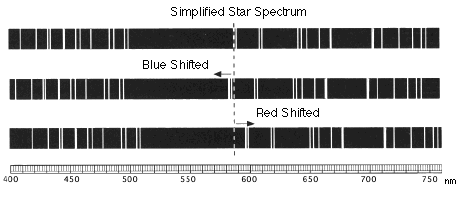
What Are Redshift And Blueshift Space




A Fluorescence Emission Spectra Of The Cds At Different Excitation Download Scientific Diagram




554 Questions With Answers In Emission Science Topic



0 件のコメント:
コメントを投稿