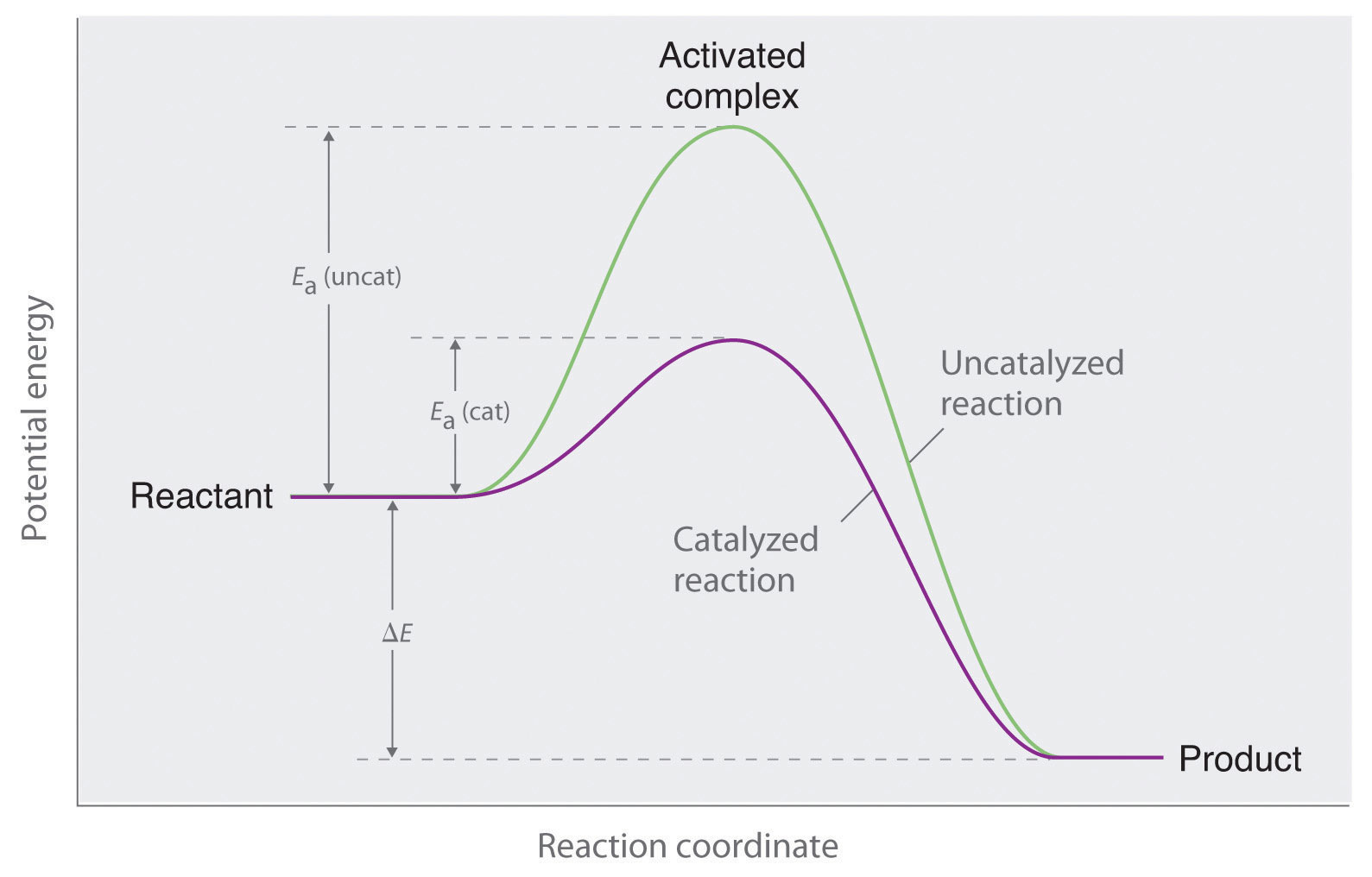
Chemical Kinetics
Presentation 2 TRAILBLAZERALZ 2 A Phase 3 Study to Assess Safety and Efficacy of Donanemab in Early Symptomatic Alzheimer's Disease, Paul Solomon 1, Jennifer Zimmer 2, Cynthia D Evans 2, Ming Lu 2, John R Sims 2, Dawn A Brooks 2, Mark A Mintun 2,3 (1 Boston Center for Memory, Boston, MA, (United States), 2My Engineering Math An unknown radioactive element decays into nonradioactive substances
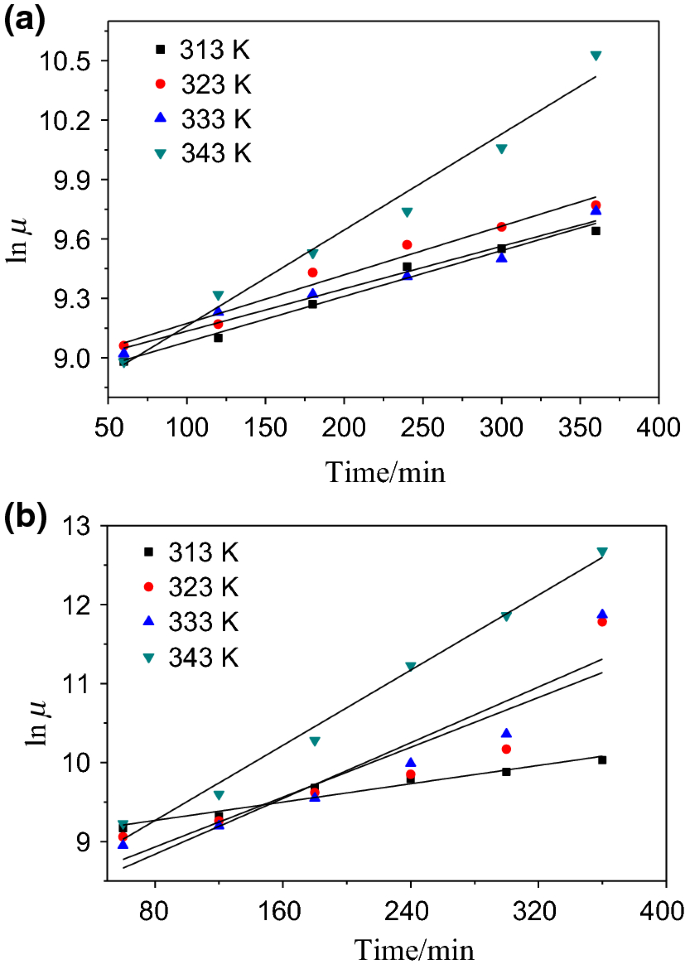
T1/2 for first order reaction is 14.26 minutes
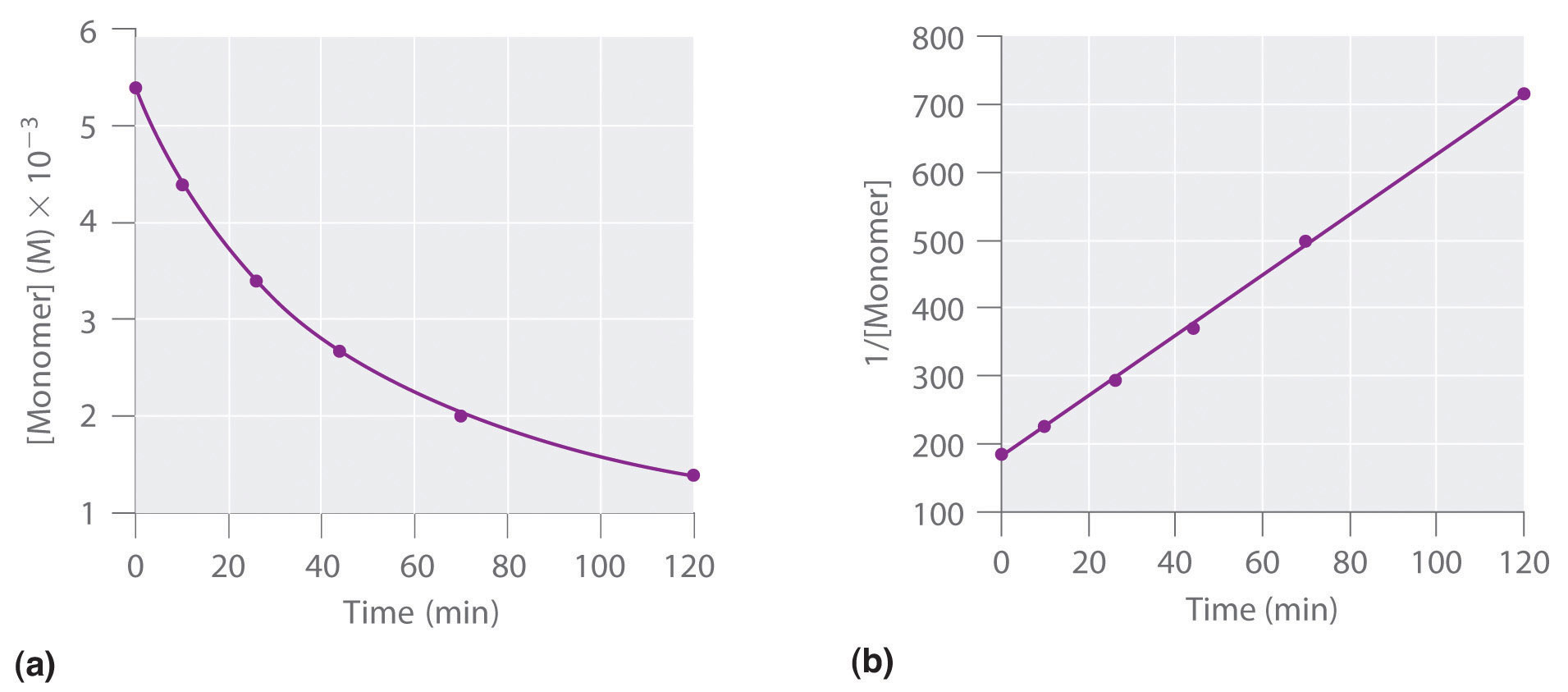
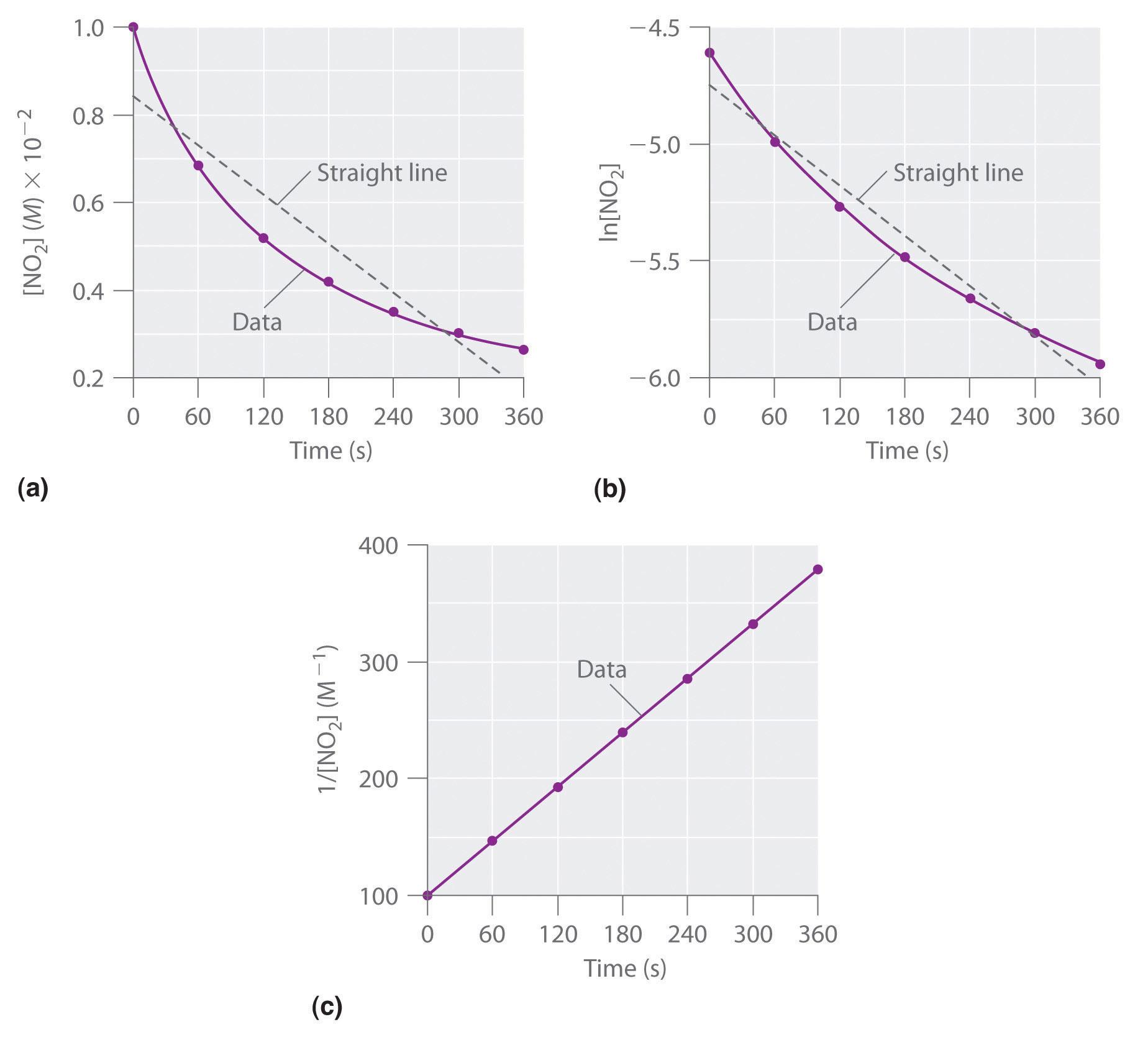
T1/2 for first order reaction is 14.26 minutes-They are provided as NO 2 , ln NO 2 , and 1/ NO 2 versus time to correspond to the integrated rate laws for zeroth, first, and secondorder reactions, respectively If a first order perception of green is represented *as* a first order perception of red, then what it will be like will be precisely how it's represented *as* If the first order state happens to be made out of exactly one trillion molecules, this won't figure in what it's like unless something about one trillion molecules figures into

Handbook Of Food Engineering
T1/2 = ln2 / k k = ln (2) / 57 = 1212 x10^4 year^1The two components of Ψ are the wave functions of the particle with spin projections 1/2 and −1/2, respectively The supersymmetric Hamiltonian is 8 Here and below, σ 1,2,3 denote the Pauli matrices, W ′ ( x) = dW / dx, etcProblem The hydrolysis of the sugar sucrose to the sugars glucose and fructose, C12H22O11 H2O C6H12O6 C6H12O6 follows a firstorder rate equation for the disappearance of sucrose Rate = kC12H22O11 (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules) k = 21 × 10−11
The halflife of a firstorder reaction is a constant that is related to the rate constant for the reaction t 1/2 = 0693/k Radioactive decay reactions are firstorder reactions The rate of decay , or activity , of a sample of a radioactive substance is the decrease in the number of radioactive nuclei per unit time8 Whites, 1 Asian, 1 Hispanic or Latino, and 1 Black or African‐American) aged 293 ± 136 years with 162 ± 23 years of schooling, who were evaluated 2‐12 weeks following concussion (range 18‐127 days, mean ±SD 51 ± 38 days, median ±IQR 45 ± 40 days postconcussion)My answer is 2 The halflife of a secondorder reaction is 2508 days How many days will have elapsed after 8 halflives?
T1/2 for first order reaction is 14.26 minutesのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
4 Solutions To Exercises | 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises | 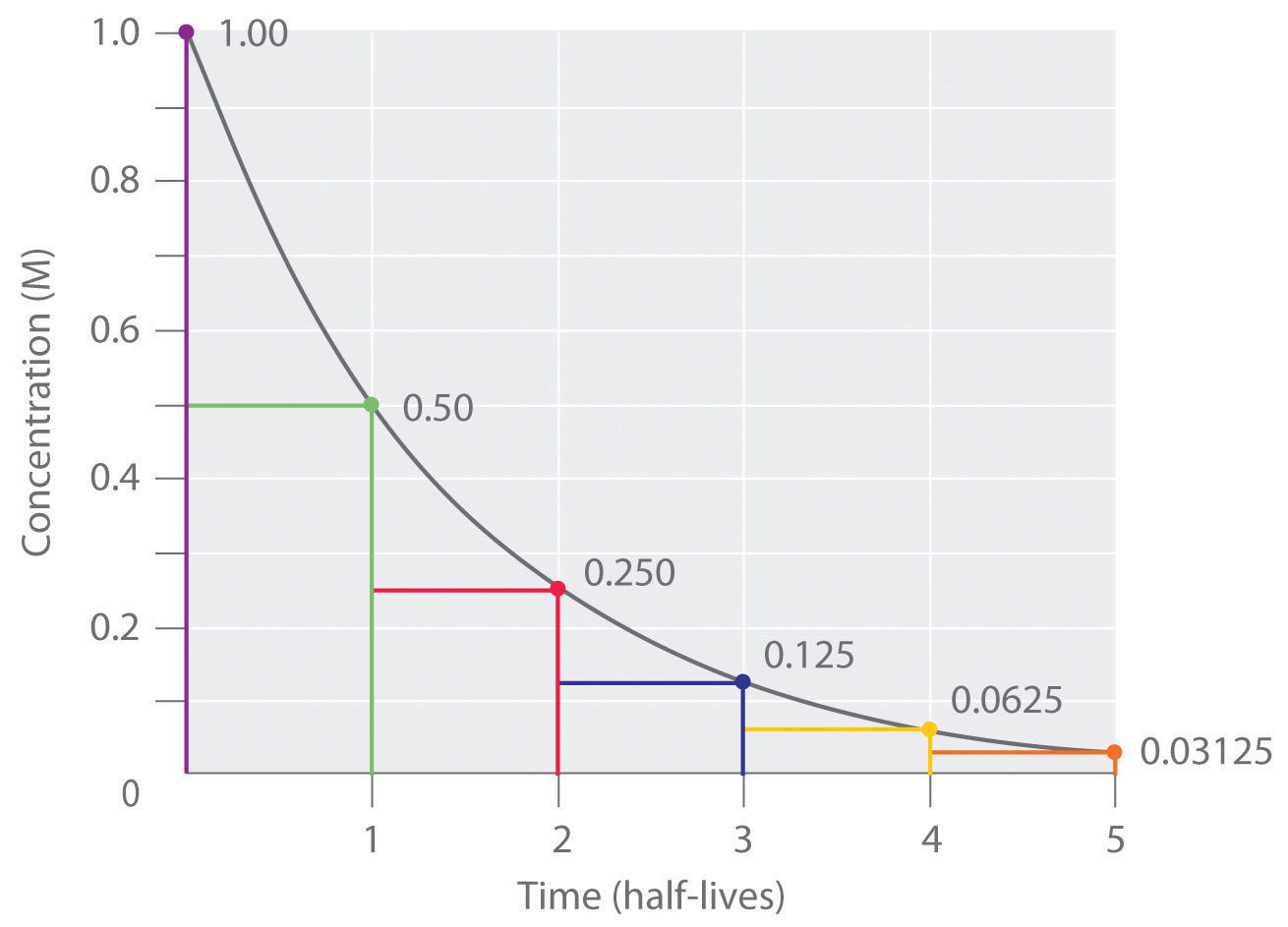 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 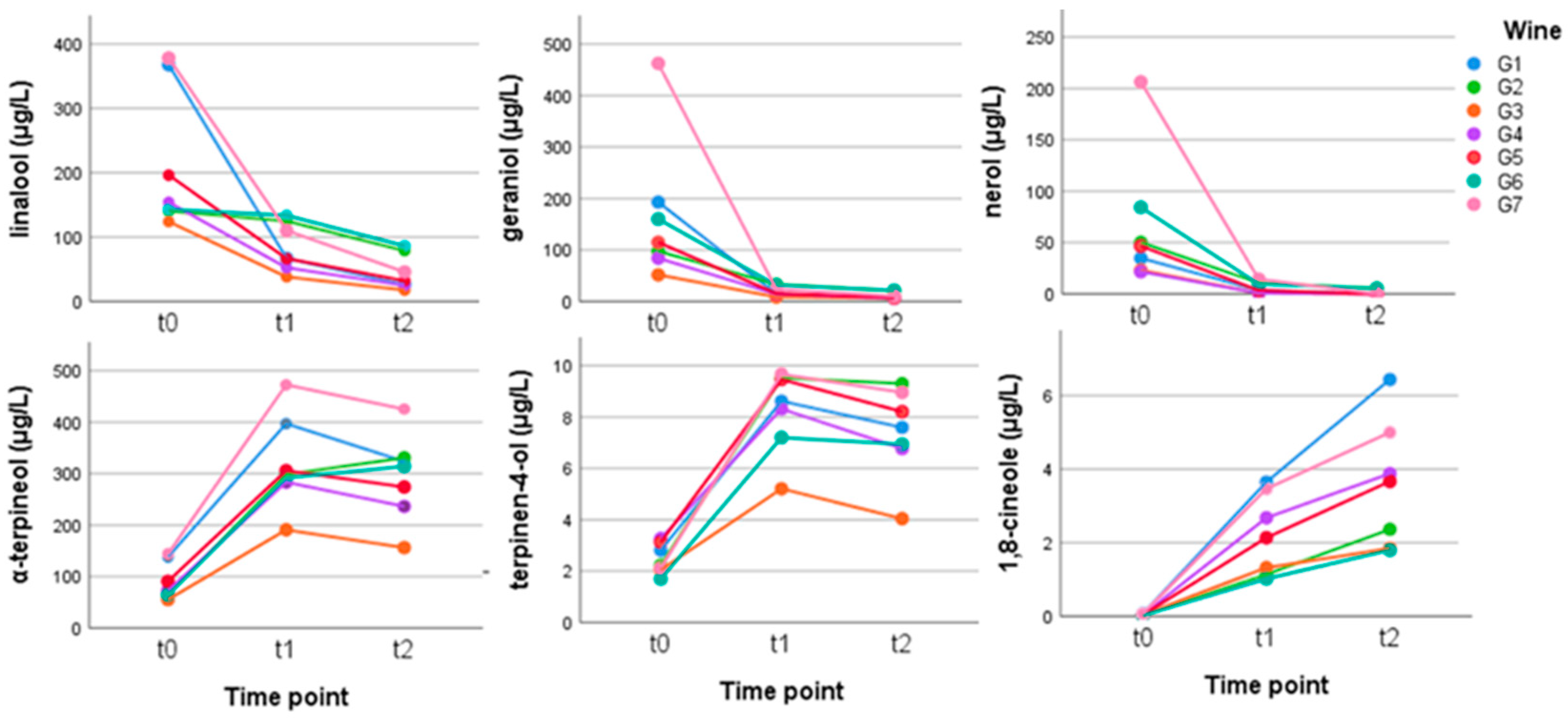 4 Solutions To Exercises |
4 Solutions To Exercises | 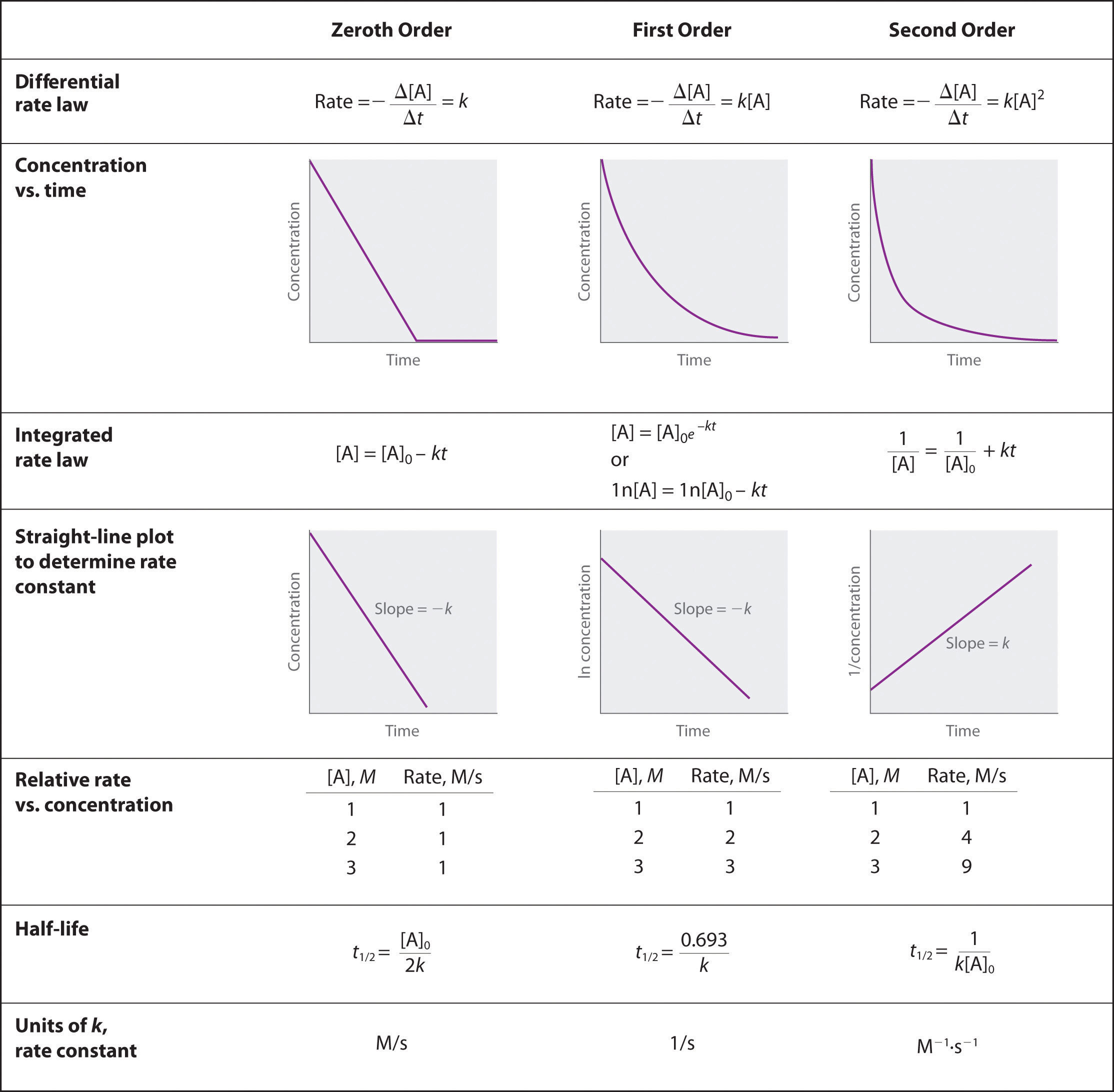 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises | 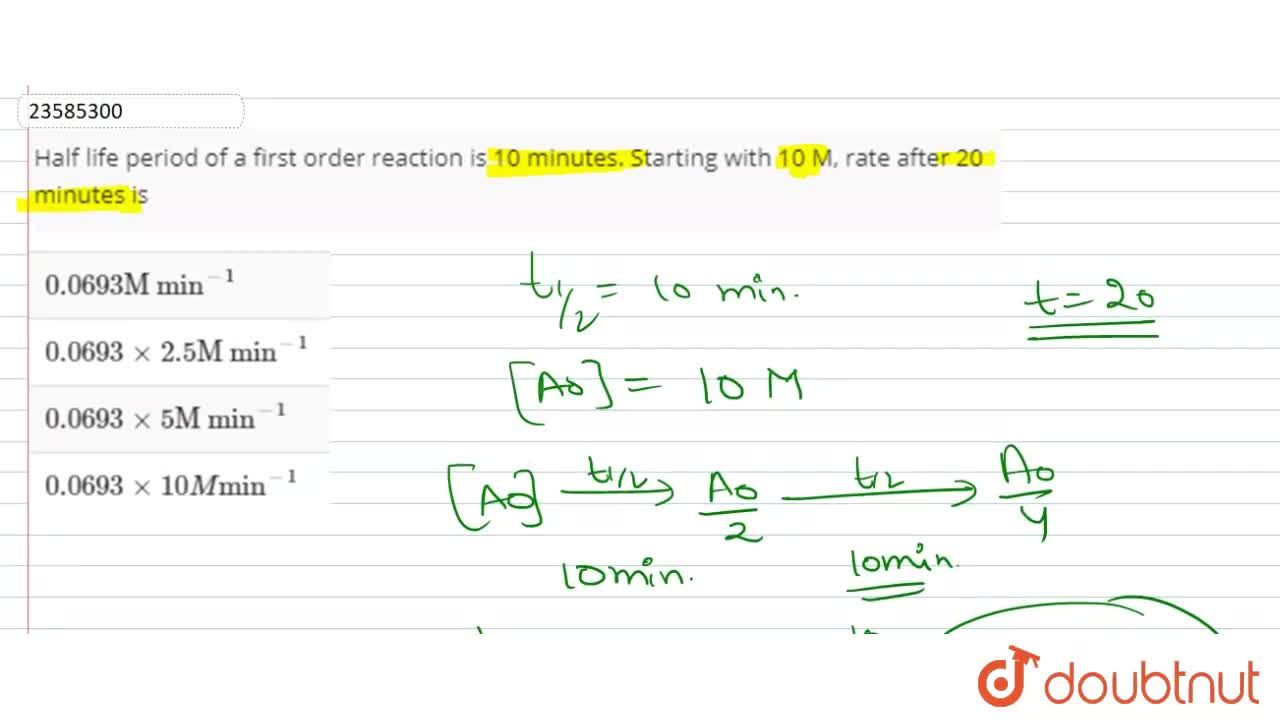 4 Solutions To Exercises |  4 Solutions To Exercises |
4 Solutions To Exercises |  4 Solutions To Exercises | 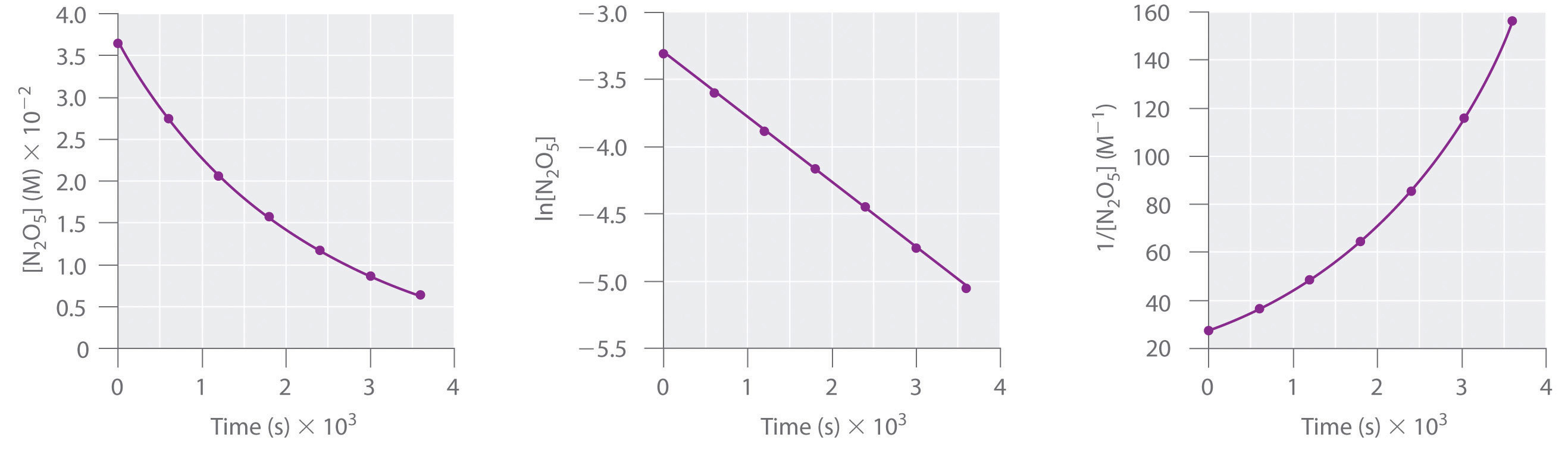 4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 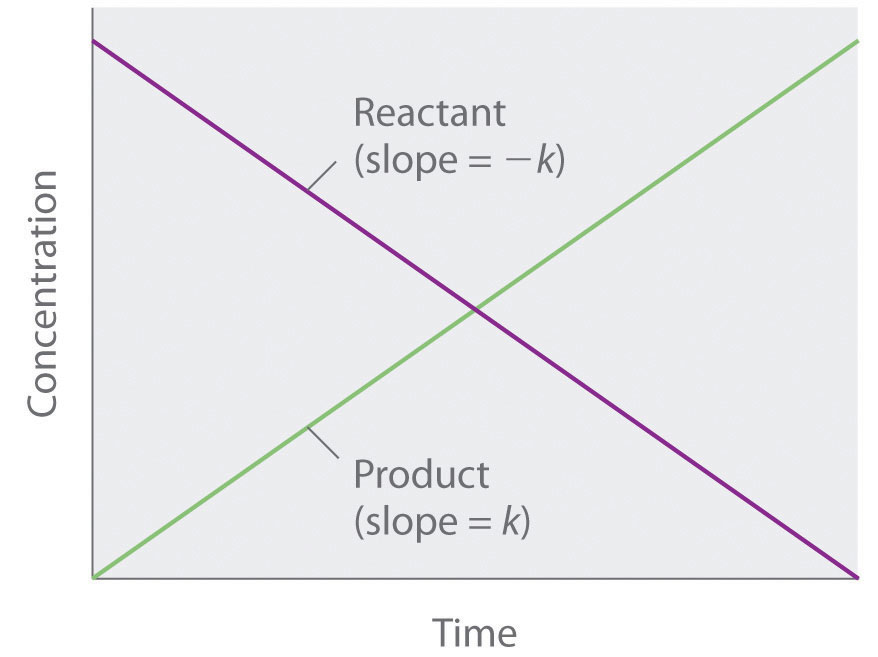 4 Solutions To Exercises |
4 Solutions To Exercises | 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
4 Solutions To Exercises | 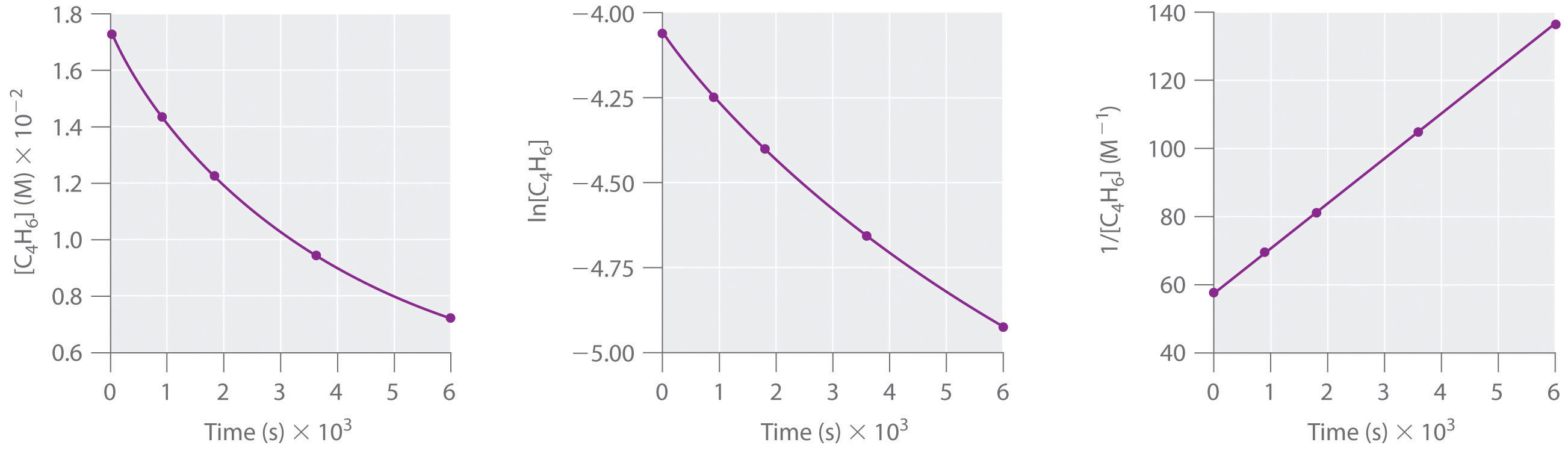 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises | 4 Solutions To Exercises | 4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
 4 Solutions To Exercises | 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
4 Solutions To Exercises | 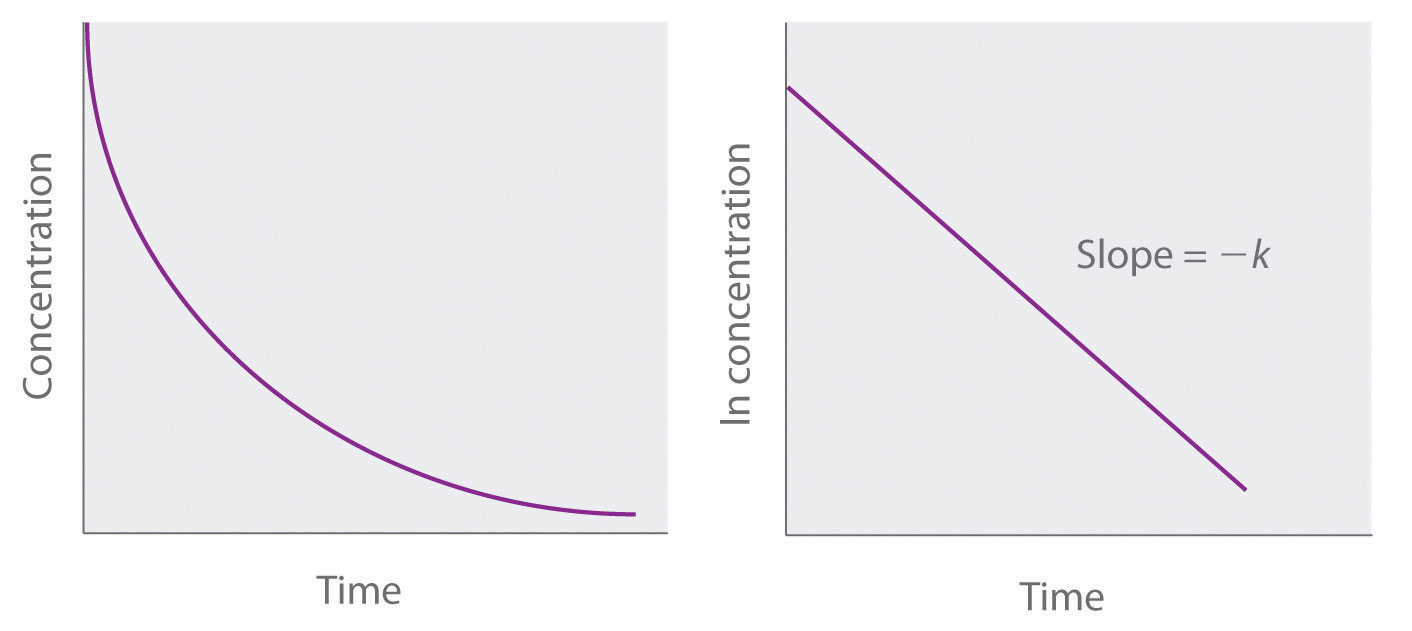 4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
4 Solutions To Exercises |  4 Solutions To Exercises |  4 Solutions To Exercises |
 4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
4 Solutions To Exercises |  4 Solutions To Exercises | 4 Solutions To Exercises |
4 Solutions To Exercises | 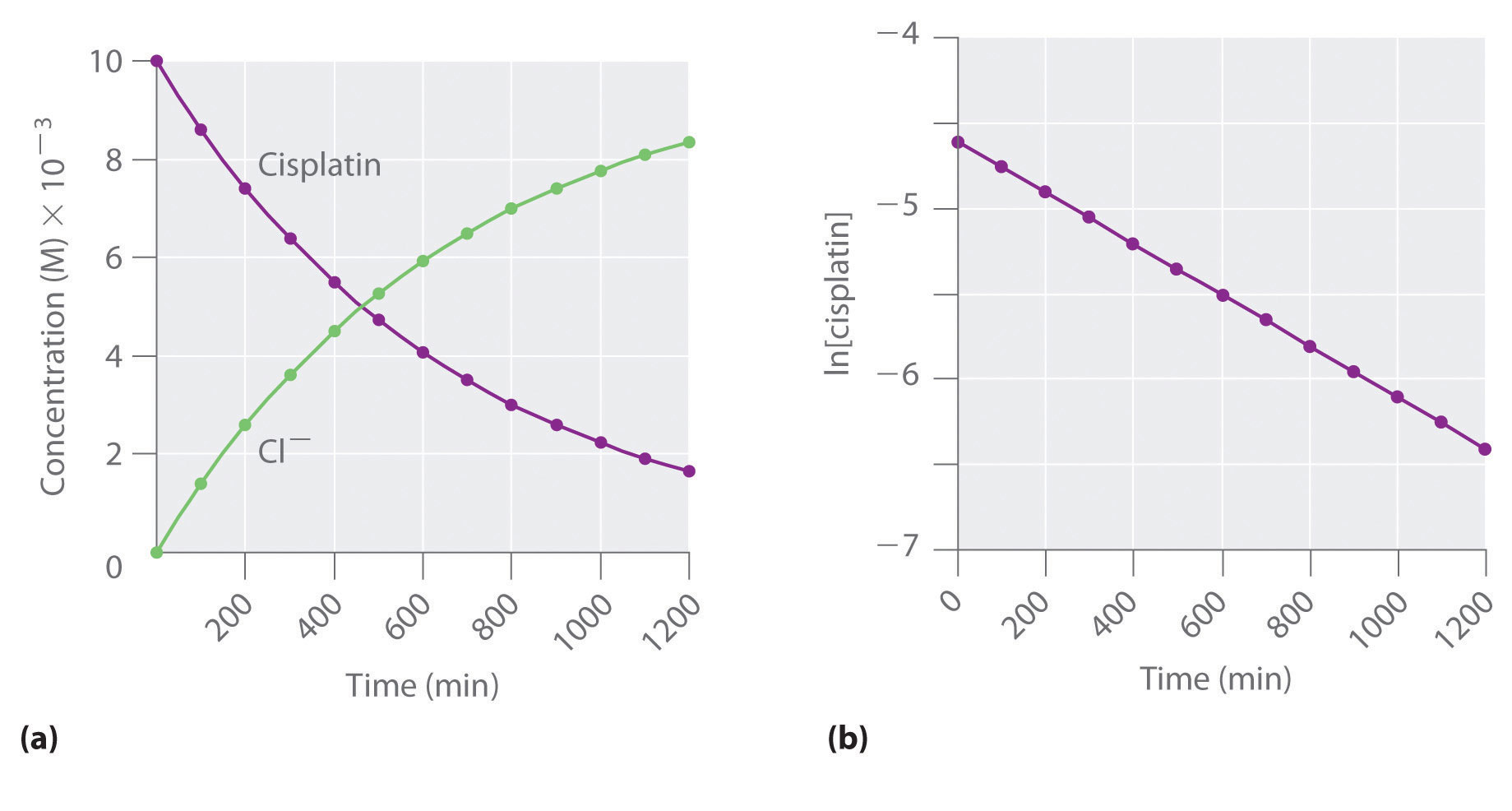 4 Solutions To Exercises | 4 Solutions To Exercises |
 4 Solutions To Exercises | 4 Solutions To Exercises |
Your Turn 1 The radioactive isotope, phosphorus32, has a halflife of 1426 days in a first order reaction What percent of phosphorus32 will remain after 60 days?A The first reaction is written as follows 6Cl2(g) 2Fe2O3(s) →4FeCl3(s) 3O2(g) Gibbs free energy





0 件のコメント:
コメントを投稿